Astyanax jordani
(Hubbs and Innes 1936) Buckup 2003

| ORDER | SUB-ORDER | FAMILY |
|---|---|---|
| Characiformes | Characoidei | Characidae |
Note
The status of the hypogean populations of Astyanax species in San Luis Potosi and Tamaulipas, Mexico, and the consequences for taxonomy and nomenclature, have been debated since their discovery in 1936. The problems have been exacerbated because there are no modern taxonomic or systematic studies of the genus. Although originally included in a separate genus (Anoptichthys) most references to these animals called them either Astyanax mexicanus (de Filippi 1853) or Astyanax fasciatus (Cuvier 1819). This was in recognition that the hypogean and epigean populations are able to interbreed, a tenet of the Biological Species Concept. There are however many important autapomorphies in the hypogean populations and, under the Phylogenetic Species Concept, the hypogean and epigean animals are separate species. This debate will continue, but I have decided here to follow my own instincts and include the hypogean forms as a separate species. This decision is supported in the most recent authoritative account of the genus (Buckup 2003). In this account A. jordani is treated as a valid separate species, and is not placed in the synonymy of either A. fasciatus or A. mexicanus. Numerous other authors (e.g. Reddell 1981; Trajano 2001) accept that the hypogean and epigean fishes belong to different species. See also the discussion of this topic in Kullander (1999:339-340) which also concludes that the epigean and hypogean populations must be considered as separate species. Two of the most recent reviews of the biology of the fishes (Wilkens and Strecker 2017, Elliott 2018) also draw this conclusion.
There are complications ahead, as it is very likely that more than one invasion of caves from the surface has taken place and that more than one species is now present in the caves of the area. However, at least one of these (from the type locality, Cueva Chica) will be validly Astyanax jordani. The others will obtain names when the situation is better understood.
Synonyms
Anoptichthys jordani Hubbs and Innes 1936
Anoptichthys antrobius Alvarez 1946
Anoptichthys hubbsi Alvarez 1947
Astyanax fasciatus (Cuvier 1819) cave form
Astyanax mexicanus (De Filippi 1853) cave form
These are the only true synonyms. Since the cave fish has often been regarded as conspecific with Astyanax mexicanus (de Filippi 1853), and with Astyanax fasciatus (Cuvier 1819), it has often been referred to under these names. Reddell (1981:238 243, 325) provides a list of names used and a full bibliography to that date.
Country
MéxicoTypes
Holotype of Anoptichthys jordani: UMMZ 113514, adult 51mm SL. Paratypes of Anoptichthys jordani: UMMZ 114486, 4 specimens; BMNH 1951.12.3:14‑17; plus others.
Distribution
Type locality: La Cueva Chica, San Luis Potosí, México. Sites from which this species has been recorded are distributed from 21o50’ – 23o10’N, 98o50’ – 99o14’W, see Mitchell, Russell and Elliott (1977) and Elliott (2018) for full details. Caves in which the fishes are found in seven areas:
| Area and cave | Length (m) | Depth (m) |
| Gómez-Farías area | ||
| Sótano de Jineo + | 302 | 144 |
| Sótano del Molino | 658 | 138 |
| Sótano Escondido + | 100 | 148 |
| Chamal-Ocampo area | ||
| Bee Cave + | 245 | 119 |
| Sótano de Caballo Moro | 285 | 211 |
| Sótano de Vásquez | 1500 | 277 |
| Northern Sierra de El Abra | ||
| Cueva de El Pachón | 1000 | 8 |
| Sótano del Venadito + | 3663 | 206 |
| Yerbaniz cluster | ||
| Sótano de Yerbaniz | 2238 | 97 |
| Sótano de Matapalma | 1722 | 86 |
| Sótano de Japonés | 4500 | 140 |
| Los Sabinos area | ||
| Sótano del Tigre + | 3000 | 162 |
| Sótano de la Roca + | 20 | 42 |
| Cueva de Los Sabinos | 1502 | 96 |
| Sótano del Arroyo | 7202 | 133 |
| Sótano de la Tinaja % | 4502 | 82 |
| Sótano de Soyate + | 206 | 234 |
| Sótano de Pichijumo (Montecillos) | 1330 | 82 |
| Sótano de Jos + | 338 | 85 |
| Sótano de las Piedras | 405 | 52 |
| Sótano de la Palma Seca | 164 | 54 |
| Southern Sierra de El Abra | ||
| Cueva de la Curva | 214 | 19 |
| Sótano de Toro | 66 | 5 |
| La Cueva Chica * | 302 | 19 |
| Los Cuates + | 400 | 33 |
| Cueva Chiquitita | 20 | 16 |
| Cueva de Otates + | 269 | 15 |
| Micos and Tamasopo areas | ||
| Cueva del Río Subterráneo | 475 | 32 |
| Cueva del Otates | 269 | 15 |
| Cueva Lienzo + | 225 | 23 |
| Cueva del Fraile + | 50 | 10 |
The known distribution of Astyanax jordani. * type locailty, % type locality of Troglomexicanus perezfarfantae (Villalobos 1971) (Crustacea: Decapoda, Palaemonidae), + caves not yet sampled for genetic studies and therefore of high priority to do so. Information from Elliott (2018)
Habitat
Most of the populations are known from lakes and streams within the vadose region of the caves. Two exceptions to this are the populations in Sótano de Soyate and Sótano del Venadito which are almost certainly living in the low level phreatic zone of the karst area. In particular the Soyate population is in a huge deep lake-like area which is probably the main groundwater flow in this area. During high flow the water levels rise considerably and fishes from the phreatic populations may become washed into vadose areas and become stranded there for a time.
Astyanax jordani is sympatric with four troglobitic invertebrate species which may form part of its food supply:
Insecta: Zygentoma: Nicoletiidae
Anelpistina quinterensis (Paclt 1979)
Synonym Neonicoletia quinterensis Paclt 1979
Crustacea: Mysidae
Speleomysis quinterensis (Villalobos 1951)
Synonym Typhlolepidomysis quinterensis Villalobos 1951
Crustacea: Decapoda
Troglomexicanus perezfarfanteae (Villalobos 1971)
Synonym Troglocubanus perezfarfantae Villalobos 1971
Crustacea: Isopoda
Speocirolana pelaezi Bolivar y Pieltain 1950
Systematics
This fish is the most extensively studied of cave dwelling animals. The reason for its pre-eminence is that it is easy to breed in captivity and interfertile with the epigean Astyanax mexicanus. These properties allow very detailed studies, and in particular genetical ones, to be made easily. A large number of publications have resulted from these studies (see Elliott (2018) and this web site for bibliographies) but there has been great confusion as to the name and status of the animal. The following discussion outlines the problem and poses a solution.
The first population to be discovered, in La Cueva Chica, was described by Hubbs and Innes (1936) as a new genus and species, Anoptichthys jordani. In the following ten years two other populations were discovered in La Cueva de El Pachon and La Cueva de Los Sabinos. Alvarez (1946, 1947) included them in the genus Anoptichthys as Anoptichthys antrobius and Anoptichthys hubbsi respectively. It is now known that the cave fishes are widespread (Mitchell, Russell, and Elliott 1977), (Reddell 1981:238‑243, 325), (Elliott 2018) and there are now 31 known sites.
In their original description of Anoptichthys jordani Hubbs and Innes (1936) recognised that: "Anoptichthys agrees with the genus and subgenus Astyanax in all apparent characters other than those associated with blindness and subterranean life". The epigean Astyanax mexicanus (for a discussion of the Astyanax mexicanus / Astyanax fasciatus “problem” see Miller and Smith 1986) and the hypogean Anoptichthys jordani are not only very similar in morphology, but they possess the same (or very similar) karyotype (Kirby, Thompson, and Hubbs 1977) and are interfertile. These, plus the sympatric distribution, provide strong evidence that Anoptichthys jordani is very closely related to, and in all probability, directly evolved from, Astyanax mexicanus. There is therefore no reason for recognising a distinct genus for the cave fishes. (See Greenwood (1976), Roberts and Stewart (1976), and Banister and Bunni (1980) for discussions of this philosophy). So, should the cave and surface fishes be regarded as members of the same species (with specifically adapted local races) or as distinct subspecies or species within the genus Astyanax ? Most authors have uncritically accepted that they are conspecific because of the interfertility. However there are a number of trenchant differences between the epigean and hypogean populations. They differ in fright reaction (Pfeiffer 1977), feeding behaviour (Schemmel 1980), the distribution and density of the taste buds (Schemmel 1980), metabolic rate (Huppop 1986), the neuromasts of the lateral line (Teyke 1990) and in competitive abilities within the cave environment (Wilkens and Hüppop 1986). Although the epigean fishes can survive and breed in darkness (contrary to the evidence of Rasquin and Rosenbloom 1954) they are out-competed by the better adapted cave fish (Wilkens and Hüppop 1986). It is certain that the hypogean fishes could neither compete nor survive on the surface. The genetic distance (as calculated by Nei's D parameter) is also relevant to this discussion. Values for D of 0.142 (Pachon population) and 0.105 (Sabinos population) (Chakraborty and Nei 1974) fall within the ranges for both subspecies (0.004 ‑ 0.351) and species (0.004 ‑ 3.000) and above that for local races (0.000 ‑ 0.049) as given by Nei (1987). A simple reading of the biological species concept (Mayr 1970:12) would confine both surface and cave fishes to the same species as a result of their interfertility. Rosen (1979:275‑278; see also Kottelat (1997:10-20)) has argued however that the biological species concept is worthless as a determiner of relationships since its primary definer, reproductive compatibility, is a primitive (plesiomorphic) attribute of members of a lineage. It therefore has no power to specify relationships within a genealogical framework. In the present case it is obvious that the cave form exhibits a number of autapomorphies (see above). The two forms should therefore be considered as good separate sister species which have not yet attained reproductive isolation.
The taxonomic consequences of this would be: one species of cave‑dwelling fish Astyanax jordani (Hubbs and Innes 1936) with one junior objective synonym, Anoptichthys jordani Hubbs and Innes 1936, and two junior subjective synonyms, Anoptichthys antrobius Alvarez 1946 and Anoptichthys hubbsi Alvarez 1947.
The above discussion is based principally on morphology. Recent work by Richard Borowsky (e.g. Borowsky 1994, 1996; Borowsky and Espinasa 1997; Borowsky and Wilkens 2002; Espinasa and Borowsky 2000, 2001), indicates that there may have been at least three independent invasions of surface fishes all of which now exhibit similar troglomorphic facies. If this is the case, and we accept that genetic characters are useful, then there should have three different names (see Kottelat (1997) for an excellent discussion of these points). Recently Dowling, Martasian and Jeffery (2002), Strecker, Bernatchez and Wilkens (2003) and Wilkens and Strecker (2003) have also shown that some cave populations are independently derived from surface fishes.
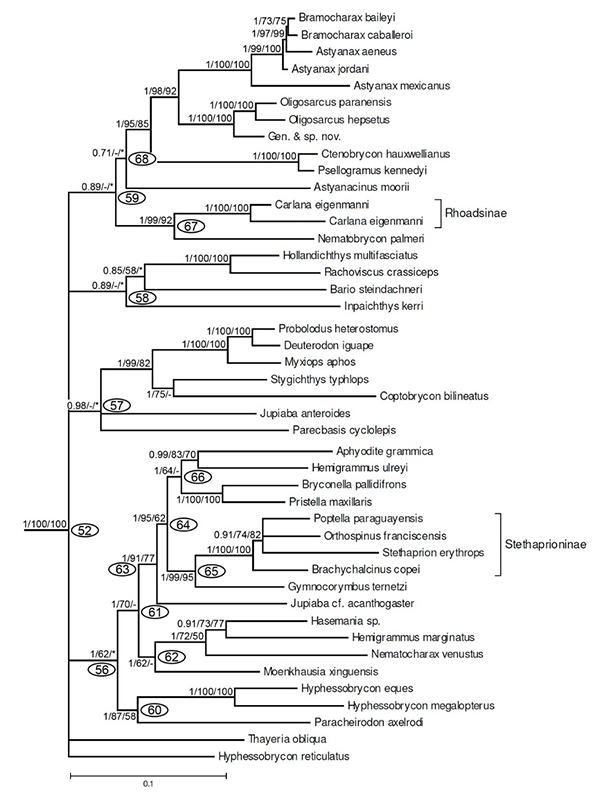
Oliveira et al. (2011) made an extensive ingroup study of the Family Characidae. Their results strongly support a sister group relationship between Astyanax jordani and a group containing Astyanax aeneus, Bramocharax caballeroi and Bramocharax baileyi (Figure below), and explicitally not a sister group relationship with Astyanax mexicanus, which is sister to the four species listed above.
The above discussion was largely written for the first edition of my book (Proudlove 2006) and needs revising in the light of much information discovered in the 14 years since it was writen. However, it is worth empasising that the conclusion of the discussion, that the cave fishes should be called Astyanax jordani, is strongly supported in the two most recent reviews of the these fishes, their habitats and their history (Wilkens and Strecker 2017:70-74 and Elliott 2018:39-44) and in an extensive phylogentic study of the Family Characidae (Oliveira et al. 2011), and also in the authoritative text on fish names by van der Laan (2019). - see phylogenetic tree below. There is a very good argument made for naming cryptic, mainly identified by molecular methods, that also applies in abundance to Astyanax jordani - see Delic et al. (2017).
Biological Notes
Here is a different justification for the cave animals being a separate species to the surface fishes.
Abstract
The most intensively studied cave animal is a characid fish from Mexico, originally described as Anoptichthys jordani. Two other species of Anoptichthys are synonyms of Anoptichthys jordani, and the genus Anoptichthys is an synonym of Astyanax. For many years the cave fishes have been considered to be conspecific with Astyanax fasciatus because the cave fishes are interfertile, under some conditions, with surface dwelling Astyanax fasciatus. In fact the cave and surface fishes are not interfertile under most, natural, conditions and are reproductively isolated by competitive exclusion. Under the tenets of the Biological Species Concept the cave fish are a separate species. Under the Phylogenetic Species Concept they are also separate species as they possess a significant number of unique character states. Since they are good species under these two species concepts they are also good species under the Evolutionary Species Concept. The cave fishes are a separate species Astyanax jordani.
Introduction
The cave-dwelling characid from Mexico is well known. It has received more study than any other cave animal and is available in pet stores and aquaria the world over. The reasons for this prominence include the facts that it is easy to breed in captivity and interfertile with the sympatric Astyanax fasciatus. These properties allow very detailed studies, and in particular genetical ones, to be made easily. A large number of publications have resulted from these studies (see Reddell, 1981 and Wilkens, 1988 for bibliographies) but there has been great confusion as to the name and status of the animal. The following discussion outlines the problem and poses a definitive solution.
The first population to be discovered, in La Cueva Chica, was described by Hubbs and Innes (1936) as a new genus and species, Anoptichthys jordani. In the following ten years two other populations were discovered in La Cueva de El Pachon and La Cueva de Los Sabinos. Alvarez (1946, 1947) included them in the genus Anoptichthys as Anoptichthys antrobius and Anoptichthys hubbsi respectively. It is now known that the cave fishes are widespread, Mitchell et al., (1977) document 29 populations (see also Reddell, 1981:238‑243, 325). The recognition of the original three populations as separate species is no longer supportable. The character states used to separate the three (details of the subdivision of the suborbital bones) is likely to be highly variable among the 29 known populations, principally because all have severe modification of the eye area as a result of eye loss during regressive evolution. In their original description of Anoptichthys jordani Hubbs and Innes (1936) recognised that: "Anoptichthys agrees with the genus and subgenus Astyanax in all apparent characters other than those associated with blindness and subterranean life". The epigean Astyanax fasciatus (previously thought to be Astyanax mexicanus, see Miller and Smith (1986) for details) and the hypogean Anoptichthys jordani are not only very similar in general morphology, but they possess the same (or very similar) karyotype (Kirby, Thompson, and Hubbs, 1977) and are interfertile under some conditions. These features, plus the sympatric distribution, provide strong evidence that Anoptichthys jordani is very closely related to, and in all probability, directly evolved from, Astyanax fasciatus. There is therefore no reason for recognising a distinct genus for the cave fishes. (See Greenwood (1976), Roberts and Stewart (1976), and Banister and Bunni (1980) for discussions of this philosophy). Since Alvarez’ species were informally synonymised with Anoptichthys jordani, and since workers dropped the genus Anoptichthys, informally considering it a junior synonym of Astyanax, the cave fishes have been most commonly referred to as the “cave form” of Astyanax mexicanus (earlier works) or A. fasciatus (more recent works). This was done on a tacit, but incorrect, acceptance of one of the tenets of the Biological Species Concept. In this paper I demonstrate that under this concept, and one other, the cave fishes are a different species from the surface fishes. The rationale for the present work is set out by Mayden and Wood (1995:110), “When entities subsumed under one binomial are actually behaving as distinct evolutionary entities, we perform no service to them nor to the biological community by treating them as a single species”.
Considering these animals as separate species is not unprecedented. Reddell (1981: 238-243), Miller and Smith (1986) and Espinosa Perez et al. (1993), in a checklist of Mexican fishes, all called the cave fish Astyanax jordani. Eschmeyer et al. (1998:816) consider that Anoptichthys jordani is valid as Astyanax jordani.
Cave populations and their relationships.
Troglomorphic Astyanax are now known from at least 29 locations in the Sierra de El Abra, Sierra de Guatemala and other areas in San Luis Potosi, Mexico (Fig. 1) (Mitchell et al., 1977). It has been suggested, on genetic grounds, that the populations vary in phylogenetic age, with some populations evolving in caves for longer than others (Wilkens, 1988). In particular, the isolated Micos population is thought to be considerably younger than most of the Sierra de El Abra populations. Borowsky and Espinasa (1997) provide DNA evidence for three separate evolutionary lines: “Northern” (Sierra de Guatemala and Nicolas Perez), “Southern” (Sierra de El Abra), and “Micos” (Fig. 2). Espinasa and Borowsky (2001) demonstrate that the southern line, which contains the type localities of all three Anoptichthys species, derives from common ancestral stock, most likely due to a single colonisation event (Fig. 3). The southern population is therefore probably a single species, separate from the other two lineages. If further work confirms that the other lineages are distinct they will require descriptions and names. The present discussion relates only to the southern populations.
Species concepts
There has recently been much interest in species concepts (reviews and discussion in Kimbel and Martin 1993, Nielsen 1995, Claridge et al. 1997, Kottelat 1997, Mayden 1999 and Wheeler and Meier 2000). Although there is still much disagreement about the “best” concept (see e.g. the debate in Wheeler and Meier 2000) Mayden (1997, 1999) has provided a significant step forward with his division of species concepts into primary and secondary. The primary, non-operational, concept is the Evolutionary Species Concept (ESC; “An evolutionary species is an entity composed of organisms that maintains its identity from other such entities through time and over space and that has its own independent evolutionary fate and historical tendencies.” (Wiley and Mayden 2000a:73)). All other concepts (Mayden (1997) discusses 25) are considered as secondary, operational, concepts. Each of these attempts to discover biodiversity in a different way. Many overlap in their methods and results whereas many others are very different in methods and results. Until this breakthrough, positions on concepts were often very entrenched to one particular concept. Mayden has shown us that each of the secondary concepts is valuable in its own right. Each will discover a portion of the total biodiversity and, used together under the over-arching primary concept, will reveal the maximum information about global biodiversity. Here I use two of the secondary concepts, the Biological Species Concept (BSC) and one version of the Phylogenetic Species Concept (PSC) to show that the cave and surface fishes are separate species.
Biological Species Concept. - “I define biological species as groups of interbreeding natural populations that are reproductively isolated from other such groups.” (Mayr 2000:17).
It is undoubtedly true that the cave fishes and surface fishes can interbreed under laboratory conditions (reference) and that F1, F2 and backcross individuals can be obtained (Wilkens 1988). It is also true that under certain conditions in the wild they can interbreed. The laboratory crosses tell us nothing as they are artificial. Interbreeding in the wild takes place only under very unusual conditions. Of 29 known sites interbreeding has only been observed in nine and of these only in three is it of significant magnitude (Mitchell et al. 1977:72-76). Under the most usual cave conditions of food scarcity the two forms cannot interbreed (Wilkens, 1988:344-347). Where surface fishes are found with cave fishes the former are so out-competed for food that they starve. In this condition they cannot produce eggs or sperm and so cannot breed. A further factor impedes successful breeding. There is more yolk in cave fish eggs (Huppop and Wilkens, 1991) and homozygous cave fish eggs are have a competitive advantage over heterozygous (cave x surface) eggs (Wilkens 1988:346). In the terms of the BCS this a pre-zygotic isolating mechanism driven by ecology and behaviour. Under unusual cave conditions where there is plenty of food the surface fishes can obtain enough and in La Cueva Chica, which has a large bat roost, the two fishes do interbreed and the Chica fish population is a hybrid one (Mitchell et al. 1977; Romero 1983). Some hybridisation has occurred in La Cueva de El Pachon (Langecker et al. 1991). In Sotano de Yerbaniz, which is food poor, large numbers of surface fishes enter the cave yet there is a very small number of hybrids (Mitchell et al. 1977:74). It is notable that the probably unrelated Micos fish “are already reproductively isolated from their epigean neighbours even though they occupy only an intermediate stage with respect to the constructive adaptations developed in phylogenetically old cave forms.” (Wilkens, 1988:346). A prime tenet of the BSC is that groups which are reproductively isolated from one another are separate species. (This is also the prime tenet of the Hennigian Species Concept, see Mayden (1997) and principally Meier and Willmann 2000)). Most cave fishes are isolated from surface fishes by competitive isolation (the competitive-exclusion principle of Mayr 1970:43-44) and therefore the cave and surface fishes are not members of the same species under the BSC. They are not forms a polymorphic species because such species, despite often great morphological variability, breed freely at contact zones (Mayr, 1970:17). They are not subspecies of A. fasciatus because subspecies are not reproductively isolated from other subspecies.
Metrical measures of similarity/difference can also be applied to the BSC where they measure “reproductive isolation and evolutionary independence” (Mayden 1997:399; the “Genetic Species Concept”). Two of these types of measure are available. Avise and Selander (1972) used electrophoresis to study allozyme variation in epigean and hypogean fishes. They use the data to calculate Rogers’ coefficient of genetic similarity (their Table 6). Similarity values for conspecific populations tend to lie in the high 0.80s and 0.90s with genetic similarity among congeneric species usually much lower, although there is overlap. Avise and Selander (1972) provide values for various congeneric species (0.61, 0.76, 0.77, 0.32, 0.21, 0.50), and various conspecific populations (0.97, 0.98, 0.88, 0.95, 0.97, 0.75, 0.89). Their calculation for the similarity between cave and surface fishes is 0.82. While this is not so low as the congeneric species listed, it is intermediate between these values and the predominant 0.90s of the conspecifics. It is as we would expect of species in the process of separation. This intermediate value does not unequivocally support the stance, often stated (e.g. Wilkens 1988:273, Romero 1983), that cave and surface fish are conspecific. Chakraborty and Nei (1974) calculated Nei’s standard genetic distance (Nei's D parameter) between epigean and hypogean fishes. Values for D of 0.142 (Pachon population) and 0.105 (Sabinos population) fall within the ranges for both subspecies (0.004 ‑ 0.351) and species (0.004 ‑ 3.000) and significantly above that for local races (0.000 ‑ 0.049) as given by Nei (1987:241-242). Neither of these measures provide unequivocal support for the fishes being separate species since they are intermediate between fully separated species and populations within a species. However we are sure that the cave fishes evolved from the surface fishes and these values show us that this process is either still occurring or only recently stopped. They certainly are not supportive of a single, genetically unified species.
Interfertility under some, rather unusual and atypical, conditions (i.e. absence of total reproductive isolation) has long been used to confine the two type of fishes into one species. The above discussion should finally lay to rest this misguided notion. Wiley and Mayden (2000b:157-158) put the case in context: “As ichthyologists, we know of no recently evolved and closely related species of North American freshwater fish that is 100% reproductively isolated from its sister species. What do we mean by closely related ? We mean species pairs that are young enough and whose biogeographic relationships are such that there is no reason to think that there is an extinct sister species left out of the analysis ... We can produce F1 hybrids if we have a mind to do so.”
Phylogenetic Species Concept. - There are a number of different formulations of the PSC. Mayden (1997) identifies three and two are discussed in Wheeler and Meier (2000). Here I use the definition of Wheeler and Platnick (2000) which is also that of Cracraft (1997), both of which stem from the studies and thoughts of Eldredge and Cracraft (1980) and Nelson and Platnick (1981). This is the PSC1 of Mayden (1997).
“We define species as the smallest aggregation of (sexual) populations or (asexual) lineages diagnosable by a unique combination of character states.” (Wheeler and Platnick 2000:58)
The cave fishes have a significant number of very distinctive and diagnosable character states (Table 1). They are quite clearly a different species from the surface fishes under this version of the PSC.
Evolutionary Species Concept. -
The cave fishes clearly “maintain their identity” and have their own “independent evolutionary fate” within the caves of central Mexico. They are therefore good Evolutionary Species.
Discussion
There is debate among those studying speciation about the extinction of ancestors at a speciation event. One view (e.g. Meier and Willmannn, 2000) is that ancestors, also called stem species, must become extinct when species evolve from them. The present case provides strong, empirical, evidence that this cannot be the case. The presence of an evolved, or evolving, cave species (Astyanax jordani), over a small part of the range of the species it is evolved, or evolving, from (Astyanax fasciatus), can in no way affect the realness of the species Astyanax fasciatus. It exists as a perfectly good species, totally unaltered over the majority of its range, despite the evolution of a new species from it in a part of Mexico.
Why is the “cave form” not an evolutionarily significant unit (ESU) of Astyanax fasciatus ? There is no consensus on the definition of an ESU (see Cracraft 1997:332-335 for discussion) and Cracraft suggests that “its objective use is virtually precluded” because of this. The prime reason for not recognising the cave fishes an ESU of Astyanax fasciatus is that any group which is sufficiently distinct to be considered as ESU is, prima facie, a distinct species under at least the PSC. There is no reason to subdivide “species” into smaller units if it can be shown that such “species” consists of groups which have an “independent evolutionary fate”. The PSC and ESC show us how to determine species and there is no justification for defining sub- or infra- specific groupings.
Taxonomic consequences
The final taxonomic position is: one species of cave‑dwelling fish Astyanax jordani (Hubbs and Innes, 1936) with one junior objective synonym, Anoptichthys jordani Hubbs and Innes, 1936, and two junior subjective synonyms, Anoptichthys antrobius Alvarez, 1946 and Anoptichthys hubbsi Alvarez, 1947. The genus Anoptichthys Hubbs and Innes, 1936 is a synonym of Astyanax Baird and Girard, 1854.
Conservation implications
There is little doubt that subpopulations exist. Mitchell et al. (1977:76-79) document that animals from eight caves are discreet when subject to a morphometric analysis. The recognition of Astyanax jordani as separate from Astyanax fasciatus does not preclude the existence of subpopulations (demes) within the jordani clade. Continued genetic studies, or detailed morphological analysis, will reveal these. Once revealed we need to determine the relevant level of protection.
References
Alvarez, J. 1946. Revision del genero Anoptichthys con descripcion de una especia nueva (Pisces, Characidae). An. Esc. Nac. Cien. Biol. Mex. 4:263-282.
Alvarez, J. 1947. Descripcion de Anoptichthys hubbsi caracinido ciego de la Cueva de Los Sabinos. S. L. P. Rev. Soc. Mex. Hist. Nat. 8:215-219.
Avise, J.C. and Selander, R.K. 1972. Evolutionary genetics of cave-dwelling fishes of the genus Astyanax. Evolution 26:1-19.
Banister, K.E. and M.K. Bunni. 1980. A new blind fish from Iraq. Bull. Br. Mus. Nat Hist. (Zool.) 38:151-158.
Borowsky, R. and L. Espinasa. 1997. Antiquity and origins of troglobitic Mexican tetras, Astyanax fasciatus. Proc. 12th Int. Cong. Speleol. 3:359-361.
Chakraborty, R. and M. Nei. 1974. Dynamics of gene differentiation between incompletely isolated populations of unequal sizes. Theor. Pop. Biol. 5:460-469.
Claridge, M.F., H.A. Dawah and M.R. Wilson. 1997. Species. The units of biodiversity. Systematics Association Special Volume Series 54. Chapman and Hall, London, UK.
Cracraft, J. 1997. Species concepts in systematics and conservation biology - an ornithological viewpoint, p. 325-339. In: Species: The units of biodiversity. The Systematics Assoc. Spec. Vol. Ser. 54. Claridge, M.F., H.A. Dawah and M.R. Wilson (eds.).
Eldridge, N. and J. Cracraft. 1980. Phylogenetic analysis and the evolutionary process. Columbia University Press, New York.
Erckens, W. and W. Martin. 1982. Exogenous and endogenous control of swimming activity in Astyanax mexicanus (Characidae, Pisces) by direct light response and by a circadian oscillator. II. Features of time-controlled behaviour of a cave population and their comparison to an epigean ancestor form. Z. Naturforsch. 37:1266-1273.
Eschmeyer, W.N., C.J. Ferraris, M.D. Hoang and D.J. Long. 1998. Species of fishes, p. 25-1820. In: Catalog of fishes. California Academy of Sciences. W. N. Eschmeyer (ed.).
Espinasa, L. and R. Borowsky. 2001. Origins and relationships of cave populations of the blind Mexican tetra, Astyanax fasciatus, in the Sierra de El Abra. Env. Biol. Fishes.
Espinosa Perez, H., M. T. Gaspar Dillanes and P. Fuentes Mata. 1993. Listados fuanisticos de Mexico III. Los peces dulceacuicolas Mexicanos. Univ. Nacional Autonoma de Mexico.
Fricke, D. 1987. Reaction to alarm substance in cave populations of Astyanax fasciatus (Characidae, Pisces). Ethology 76:305-308.
Greenwood, P.H. 1976. A new and eyeless cobitid fish (Pisces, Cypriniformes) from the Zagros Mountains, Iran. J. Zoology, Lond. 180:129-137.
Hubbs, C.L and W.T. Innes. 1936. The first known blind fish of the family Characidae: A new genus from Mexico. Occ. Pap. Mus. Zool. Univ. Mich. No. 342:1-7.
Huppop, K. 1987. Food-finding ability in cave fish (Astyanax fasciatus). Int. J. Speleol. 16:59-66.
Huppop, K. 1988. Phanomene und Bedeutung der Energieersparnis beim Hohlensalmler Astyanax fasciatus. Doctoral Thesis, University of Hamburg.
Huppop, K. and H. Wilkens. 1991. Bigger eggs in subterranean Astyanax fasciatus (Characidae, Pisces). Z. zool. Syst. Evolut.-forsch. 29:280-288.
Kimbel W.H. and L.B. Martin. 1993. Species, species concepts and primate evolution. Plenum Press, New York and London.
Kirby, R.F. K.W. Thompson and C. Hubbs. 1977. Karyotypic similarities between the Mexican and blind tetras. Copeia 1977:578-580.
Kottelat, M. 1997. European freshwater fishes. An heuristic checklist of the freshwater fishes of Europe (exclusive of former USSR) with an introduction for non-systematists and comments on nomenclature and conservation. Biologia, Bratislava 52/Suppl. 5:1-271.
Langecker, T.G., H. Wilkens and P. Junge. 1991. Introgressive hybridisation in the Pachon cave population of Astyanax fasciatus (Teleostei: Characidae). Ichthyol. Explor. Freshwaters 2:209-212.
Mayden, R. 1997. A hierarchy of species concepts: the denouement in the saga of the species problem, p. 381-424. In: Species: The units of biodiversity. The Systematics Assoc. Spec. Vol. Ser. 54. Claridge, M.F., H.A. Dawah and M.R. Wilson (eds.).
Mayden R. 1999. Consilience and a hierarchy of species concepts: Advances toward closure on the species puzzle. J. Nematol. 31:95-116.
Mayden, R. and R.M. Wood. 1995. Systematics, species concepts and the evolutionary significant unit in biodiversity and conservation biology. Amer. Fish. Soc. Symp. 17:58-113.
Mayr, E. 1970. Populations, species and evolution. The Belknap Press of Harvard University Press, Cambridge, USA.
Mayr, E. 2000. The Biological Species Concept, p. 17-29. In: Species concepts and phylogenetic theory: A debate. Columbia University Press, New York, USA. Wheeler, Q.D. and R. Meier (eds.).
Meier, R. and R. Willmannn. 2000. The Hennigian species concept, p. 30-43. In: Species concepts and phylogenetic theory: A debate. Columbia University Press, New York, USA. Wheeler, Q.D. and R. Meier (eds.).
Miller, R.R. and M.L. Smith. 1986. Origin and geography of the fishes of central Mexico, p. 487-517. In: The zoogeography of North American freshwater fishes. Hocutt, H.C. and E.O. Wiley (eds.).
Mitchell, R.W., W.H. Russell and W.R. Elliott. 1977. Mexican eyeless characin fishes, genus Astyanax: Environment, distribution and evolution. Special Publications, The Museum, Texas Tech University 12:1-89.
Nielsen, J.L. 1995. Evolution and the aquatic ecosystem: defining unique units in population conservation. Amer. Fish. Soc. Symp. 17, Bethesda, Maryland.
Nei, M. 1987. Molecular evolutionary genetics. Columbia University Press, New York.
Nelson G. and N.I. Platnick 1981. Systematics and biogeography: cladistics and vicariance. Columbia University Press, New York.
Parzefall, J. 1983. Field observations in epigean and cave populations of the Mexican characid Astyanax mexicanus (Pisces, Characidae). Mem. Biospeol. 10:171-176.
Parzefall, J. 1985. On the heredity of behaviour patterns in cave animals and their epigean relatives. Bull. Nat. Spel. Soc. 47:128-135.
Reddell, J.R. 1981. A review of the cavernicole fauna of Mexico, Guatemala and Belize. Texas Mem. Mus. Bull. 27:1-327.
Roberts, T.R.and D.J. Stewart. 1976. An ecological and systematic survey of fishes in the rapids of the lower Zaire or Congo river. Bull. Mus. Comp. Zool., Harvard 147:239-317.
Romero, A. 1983. Introgressive hybridisation in the Astyanax fasciatus (Pisces: Characidae) population at La Cueva Chica. Nat. Speleol. Soc. Bull. 45:81-85.
Rose, F.L. and R.W. Mitchell. 1982. Comparative lipid values of epigean and cave-adapted Astyanax. Southwest. Nat. 27:357-358.
Schemmel, C. 1980. Studies on the genetics of feeding behaviour in the cave fish Astyanax mexicanus f. anoptichthys. An example of apparent monofactorial inheritance by polygenes. Z. Tierpsycol. 53:9-22.
Teyke, T. 1990. Morphological differences in neuromasts of the blind cave fish Astyanax hubbsi and the sighted river fish Astyanax mexicanus. Brain, Behav. Evol. 35:23-30.
Wheeler, Q.D. and R. Meier. 2000. Species concepts and phylogenetic theory: A debate. Columbia University Press, New York, USA.
Wheeler, Q.D. and N.L. Platnick. 2000. The Phylogenetic Species Concept (sensu Wheeler and Platnick), p. 55-69. In: Species concepts and phylogenetic theory: A debate. Columbia University Press, New York, USA. Wheeler, Q.D. and R. Meier (eds.).
Wiley, E.O. and Mayden, R.L. 2000a. The Evolutionary Species Concept, p. 70-89. In: Species concepts and phylogenetic theory: A debate. Columbia University Press, New York, USA. Wheeler, Q.D. and R. Meier (eds.).
Wiley, E.O. and Mayden, R.L.. 2000b. A critique from the evolutionary species concept perspective, p. 146-158. In: Species concepts and phylogenetic theory: A debate. Columbia University Press, New York, USA. Wheeler, Q.D. and R. Meier (eds.).
Wilkens, H. 1988. Evolution and genetics of epigean and cave Astyanax fasciatus (Characidae, Pisces). Support for the neutral mutation theory. Evol. Biol. 23:271-367.
Table 1. Morphological, behavioural, physiological and genetic differences between the surface (epigean) and cave (hypogean) fishes
| Character |
Character state |
Character state | |||
| Epigean | Hypogean | Populations | References | ||
| Morphology | |||||
| Taste buds | mouth and lips only | whole head | Pa, Pi, S, Y | Schemmel 1980 | |
| Neuromast area | 50x30x10-6m | 80x50x10-6m | CS | Teyke 1990 | |
| Cupula length | 42x10-6m | 100-300x10-6m | CS | Teyke 1990 | |
| Egg size | ~1mm dia. | ~1.1mm dia. | Pa, Pi, Y | Huppop and Wilkens 1991 | |
| Scales | present | present but smaller | Pa | Wilkens 1988 | |
| Eyes | present | degenerated | Pa, S, C | Wilkens 1988 | |
| Melanin pigment | present | reduced | Pa, Pi, S, Y | Wilkens 1988 | |
| Behaviour | |||||
| Fright reaction | present | reduced | C, Pa, Pi | Fricke 1988 | |
| Feeding | 90o to subsrate | 45o to subsrate | Pa, Pi, S, Y | Schemmel 1980 | |
| Food finding | 20% efficient | 80% efficient | Pa | Huppop 1987 | |
| Agressive | present | reduced | Pa, Mi | Burchards, Dolle and Parzefall 1985 | |
| Schooling | present | absent | Pi | Parzefall 1983 | |
| Circadian rhythm | normal | much modified | Pa | ||
| Physiology | |||||
| Fat storage | 9% of fresh body mas | 37% of fresh body mass | Pa | Huppop 1988 | |
| Lipids | 0.32+/-0.14* | 1.26+/-0.27* | Y | Rose and Mitchell 1982 | |
| Genetics | |||||
| Allozyme Es-2d | absent | present | C, Pa, S | Avise and Selander 1972 |
* mean lipid to lean dry weight ratio
+ C = La Cueva Chica. CS = Commercial stock. Probably derived from the Chica population. Pa = La Cueva de El Pachon. Pi = El Sotano de Las Piedras. S = La Cueva de los Sabinos. Y = El Sotano de Yerbaniz. (All are from the “southern” population). Mi = La Cueva del Rio Subterraneo (unrelated Micos population)
Conservation Status
[NE]
R (IUCN, 1990), VU A1ac+2c,B1+2c,D2 (IUCN, 1996, 2000). These criteria indicate: (A1ac+2c) that the population has been, or will be, reduced by 20% over the past 10 years (and/or over the next 10 years) as a result of habitat loss or degeneration, (B1+2c) that the area of occupancy is less than 2000 km2 and a severely fragmented population with a decline in extent or quality of habitat, (D2) that the population exists in 5 or less sites. This latter criterion is not valid as the species is known from 31 sites (Mitchell, Russell and Elliott 1977, Elliott 2018). Special Concern (Contreras-Balderas, Almada-Villela, Lozano-Vilano and Garcia-Ramirez 2003).
Museum Holdings
Anoptichthys jordani: BMNH 1973.9.10:20
Anoptichthys hubbsi: BMNH 1951.12.3:14‑17 (Paratypes)
Internet Resources
Cave Fish Adolescence Means Sprouting Taste Buds in Weird Places
A very nice film of research into Astyanax, see below for credits for this film
Tital: Poissons mexicains, un oeil sur l'évolution, Année de production : 2017, Durée : 6 min 10, Réalisateur : Nicolas Mifsud, Producteur : CNRS Images, Intervenant(s) : Sylvie Rétaux, Maryline Blin,, L'Institut de Neurosciences de Paris Saclay, CNRS / Université Paris-Sud / Université Paris-Saclay
The 6th Annual Cavefish Meeting was held March 17th-20th 2019. Links to the meeting abstracts will be added a soon as I know them.
The Astyanax research community
Astyanax developmental gene expresion
Association for Mexican Cave Studies
How and why does the cave fish lose its eyes?
Bill Elliott's Astyanax web site
Bill Elliott's chapter 1 in "Biology and evolution of the Mexican cavefish" (Keene et al. 2016)
Bill Elliott's chapter 3 in "Biology and evolution of the Mexican cavefish" (Keen et al. 2016)
Film of surface fishes from Jeffery (2020). Paper Open Access, film by Mandy Ng.
Film of troglomorphic fishes from Jeffery (2020). Paper Open Access, film by Mandy Ng.
Film of removal of lens from Jeffery (2020). Paper Open Access, film by Yoshiyuki Yamamoto.
Key References
This species has been studied in much greater detail than any other subterranean fish, and probably any other subterranean organism. Consequently the literature is very large. Anyone working with this species, or taking a serious interest in it, will need to follow the primary literature which is published across a very wide array of primary scientific journals. Those people starting out in research on Astyanax jordani, and those with a passing interest, will have to read and fully digest all of the major review publications, outlined below, all of which are required initial reading. Only by being fully familiar with these works can it be possible to move onto the primary literature with confidence that all the necessary background is firmly embedded in memory. In chronological order these are:
Mitchell, Russell and Elliott (1977) - Mexican eyeless characin fishes, genus Astyanax: Environment,distribution and evolution
Wilkens (1988) - Evolution and genetics of epigean and cave Astyanax fasciatus (Characidae, Pisces): support for the neutral mutation theory
Wilkens (1992) - Neutral mutations and evolutionary progress
Jeffery (2001) - Cavefish as a model system in evolutionary developmental biology
Fish (2004) - Karst hydrology of the Sierra de El Abra [Originally a thesis at McMaster University, Fish (1977)]
Wilkens (2005) - Fish
Jeffery (2005) - Evolution of eye degeneration in cavefish: The return of pleiotropy
Jeffery (2005) - Regressive Evolution of Pigmentation in the Cavefish Astyanax
Jeffery (2006) - Regressive Evolution of Pigmentation in the Cavefish Astyanax
Protas et al. (2007) - Regressive evolution in the Mexican cave tetra, Astyanax mexicanus
Jeffery (2008) - Emerging model systems in evo-devo: cavefish and microevolution of development
Jeffery (2009) - Regresive evolution in Astyanax cavefish
Jeffery (2009) - Evolution and development in the cavefish Astyanax
Borowsky (2010) - The evolutionary genetics of cave fishes: Convergence, adaptation and plieotropy
Jeffery and Strickler (2010) - Development as an evolutionary process in Astyanax cavefish
Wilkens (2010) - Genes, modules and the evolution of cave fish
Juan, Guzik, Jaume and Cooper (2010) - Evolution in caves: Darwin’s ‘wrecks of ancient life’ in the molecular era
Yamamota and Jeffery (2011) - Blind cavefish
Kish, Bohnsack, Gallina, Kasprick and Kahana (2011) - The eye as an organizer of craniofacial development
Jeffery (2012) - Astyanax mexicanus: A model organism for evolution and adaptation
Wilkens (2012) - Neutral mutations
Trontelj (2012) - Natural selection
Gross (2012) - The complex origin of Astyanax cavefish
Protas and Jeffery (2012) - Evolution and development in cave animals: from fish to crustaceans
Bleckmann, Mogdans and Coombs (2014) - Flow sensing in air and water. Behavioral, neural and engineering principles of operation
Windsor (2014) - Hydrodynamic imaging by blind Mexican cavefish
Niemiller and Soares (2015) - Cave environments
Gross, Meyer and Perkins (2015) - The rise of Astyanax cavefish
Keene, Yoshizawa and McGaugh (2016) - Biology and evolution of the Mexican cavefish
Casane and Retaux (2016) - Evolutionary genetics of the cavefish Astyanax mexicanus
Foulkes, Whitmore, Vallone and Bertolucci (2016) - Studying the evolution of the vertebrate circadian clock: The power of fish as comparative models
Wilkens and Strecker (2017) - Evolution in the dark: Darwin's loss without selection
Tuccinardi (2017) - Keeping blind cave fishes (or not)
Elliott (2018) - The Astyanax caves of Mexico: Cavefishes of San Luís Potosí, Tamaulipas, and Guerrero
Borowsky (2018) - Primer. Cavefish
Gore, Jeffery, Retaux and Rohner (2018) - Cavefish development. 18 paper special issue, all open access and you can dowload the whole issue as a zip file
Torres-Paz, Hyacinthe, Pierre and Retaux (2018) - Towards an integrated approach to understand Mexican cavefish evolution
Rohner (2018) - Out of the dark. Cavefish are entering biomedical research
Jeffery (2019) - Astyanax mexicanus: A vertebrate model for evolution, adaptation, and development in caves
Mogdans (2019) - Sensory ecology of the fish lateral line system: Morphological and physiological adaptations for the perception of hydrodynamic stimuli
Loomis et al. (2019) - An adult brain atlas reveals broad neuroanatomical changes in independently evolved populations of Mexican cavefish
Wilkens (2020) - The role of selection in the evolution of blindness in cave fish
Jeffery (2020) - Astyanax surface and cave fish morphs
McGaugh et al. (2020) - Dark world rises: The emergence of cavefish as a model for the study of evolution, development, behavior, and disease
Maldonado et al. (2020) - Subterranean life: Behavior, metabolic, and some other adaptations of Astyanax cavefish
Espinasa, Ornelas-Garcia, Legendre, Retaux, Best, Gamboa-Miranda, Espinosa-Perez and Spouse (2020) - Two new localities of Astyanax cavefish plus revision of its biogeography
Mercado-Silva, Ornelas-Garcia, Schmitter-Soto, Gidmark and Simons (2020) - Characidae: Characins (large book chapter review with much information on Astyanax jordani)
Kowalko, Franz-Odendaal and Rohner (2020) - Cavefish special issue of the Journal of Experimental Zoology. Part B Molecular and Developmental Evolution
Warren et al. (2021) - A chromosome-level genome of Astyanax mexicanus surface fish for comparing population-specific genetic differences contributing to trait evolution
Riddle et al. (2021) - Genetic mapping of metabolic traits in the blind Mexican cavefish reveals sex‑dependent quantitative trait loci associated with cave adaptation
Wilkens (2021) - Variability and the primacy of the genotype
Baumann and Ingalls (2022) - Mexican tetra (Astyanax mexicanus): biology, husbandry, and experimental protocols
Retaux (2022) - La boîte à outils de l’évolution développementale ou comment les poissons cavernicoles mexicains ont perdu leurs yeux
Gross, Boggs, Rétaux and Torres-Paz (2023) - Developmental and genetic basis of troglomorphic traits in the teleost fish Astyanax mexicanus
Berning and Gross (2023) - The constructive evolution of taste in Astyanax cavefish: A review
Perera, Guera and Riddle (2023) - The Mexican Tetra, Astyanax mexicanus, as a model system in cell and developmental biology
Also, not a review, McGaugh et al. (2014) - The cavefish genome reveals candidate genes for eye loss
Also useful, though mostly superceeded by Elliott (2018), are Russell and Raines (1967) and Morris (1989).
Selected bibliography
- Hubbs, C.L. and Innes, W.T. (1936)
- Hykes, O. V. (1937)
- Innes, W.T. (1937)
- Jordan, C. B. (1937)
- Bridges, W. (1940)
- Gresser, E.B. and Breder, C.M. (1940)
- Anonymous (1940)
- Breder, C.M. and Gresser, E.B. (1941)
- Breder, C.M. and Gresser, E.B. (1941)
- Breder, C.M. and Gresser, E.B. (1941)
- Breder, C.M. (1942)
- Breder, C.M. (1943)
- Breder, C.M. (1943)
- Bridges, W. (1943)
- Breder, C.M. (1943)
- Osorio Tafall, B.F. (1943)
- Breder, C.M. and Rasquin, P. (1943)
- Breder, C.M. (1944)
- Benn, J.H. (1945)
- Anonymous (1945)
- Breder, C.M. (1945)
- Rasquin, P. (1946)
- Breder, C.M. and Halpern, F. (1946)
- Alvarez, J. (1946)
- Jordan, C.B. (1946)
- Breder, C.M. and Rasquin, P. (1947)
- Breder, C.M. and Rasquin, P. (1947)
- Schlagel, S.R. and Breder, C.M. (1947)
- Rasquin, P. (1947)
- Breder, C.M. and Roemhild, J. (1947)
- Alvarez, J. (1947)
- Nigrelli R.F. (1947)
- Rasquin, P. (1949)
- Rasquin, P. (1949)
- Rasquin, P. (1949)
- Atz, J.W. (1950)
- Breder, C.M. and Rasquin, P. (1950)
- Bolivar y Pieltain, C. (1950)
- Villalobos, A. (1951)
- Rasquin, P. and Hafter, E. (1951)
- Breder, C.M. and Hafter Atz., E. (1952)
- Meder, E. (1952)
- Atz, E.H. (1953)
- Heuts, M. J. (1953)
- Luling, K.H. (1953)
- Luling, K.H. (1953)
- Luling, K.H. (1953)
- Bonet, F. (1953)
- Breder, C.M. (1954)
- Kuhn, O. and Kahling, G. J. (1954)
- Thines, G. (1954)
- Luling, K.H. (1954)
- Luling, K.H. (1954)
- Stefanelli, A. (1954)
- Stefanelli, A. (1954)
- Rasquin, P. and Rosenbloom, L. (1954)
- Luling, K.H. (1955)
- Bridges, W. (1955)
- Luling, K.H. (1955)
- Luling, K.H. (1955)
- Schutz, F. (1956)
- Barr, T. C. (1956)
- Sadoglu, P. (1956)
- Grunewald-Lowenstein, M. (1956)
- Thines, G. and Kahling, J. (1957)
- Sadoglu, P. (1957)
- Hahn, G. (1957)
- Sadoglu, P. (1957)
- Kahling, J. (1957)
- Luling, K.H. (1957)
- John, K. R. (1957)
- Stolk, A. (1958)
- Cahn, P.H. (1958)
- Grobbel, G. and Hahn, G. (1958)
- Bertin, L. (1958)
- Pelz, H. W. (1958)
- Stolk, A. (1959)
- Breder, C.M. (1959)
- Kuhn, O. (1960)
- Hahn, G. (1960)
- Humbach, I. (1960)
- Humbach, I. (1960)
- Frank, S. (1960)
- Baslow, M. H. and Nigrelli, R. F. (1961)
- Kahling, J. (1961)
- Frank, S. (1961)
- Bath, H. (1962)
- Franck, A. (1962)
- Friedman, L.R. (1962)
- Goettert, L. (1962)
- Goodrick, C.L. (1962)
- Luling, K.H. (1962)
- Pfeiffer, W. (1963)
- Burgers, A.C.J., Bennink, P.J.H. and van Oordt, G.J. (1963)
- Pfeiffer, W. (1963)
- Berra, T.M. (1963)
- John, K.R. (1964)
- Franck, A. (1964)
- Berra, T.M. (1964)
- John, K.R. and Haut, M. (1964)
- Thines, G., Wolff-Van Ermengem, F., Boucquey, C. and Soffie, M. (1965)
- Kosswig, C. (1965)
- Boucquey, C., Thines, G. and Van Der Borght, C. (1965)
- Glaser, D. (1965)
- Reed, M. (1966)
- Peters, N. and Peters, G. (1966)
- Pfeiffer, W. (1966)
- Pfeiffer, W. (1966)
- Carmignani, M. P. A. (1966)
- Cavicchioli, G. and Guarnieri, P. (1966)
- Thines, G., Soffie, M. and Vandenbussche, E. (1966)
- Reddell, J.R. (1967)
- Kosswig, C. (1967)
- Schemmel, C. (1967)
- Mattheij, J.A. and Van Oordt, P.G.W.J. (1967)
- Kuhn, O. and Strotkoetter, E. (1967)
- Reddell, J.R. (1967)
- Walters, V. and Liu, R. K. (1967)
- Johnson, K.W. (1967)
- Pfeiffer, W. (1967)
- Pfeiffer, W. (1967)
- Pfeiffer, W. (1967)
- Popper, A.N. and Tavolga, W.N. (1967)
- Sadoglu, P. (1967)
- Mattheij, J.A. (1968)
- Frank, S. (1968)
- Carmignani, M. P. A. (1968)
- Wilkens, H. (1968)
- Mattheij, J.A. (1968)
- Glaser, D. (1968)
- Sadoglu, P. and McKee, A. (1969)
- Mattheij, J.A. (1969)
- Frank, S. (1969)
- Mattheij, J.A. and Sprangers, J.A.P. (1969)
- Campos, H. (1969)
- John, K.R. and Kaminester, L.H. (1969)
- Weiss, B.A. (1969)
- Mattheij, J.A., Sprangers, J.A.P. and Van Oordt, P.G.W.J. (1969)
- Morris, R.A. (1969)
- Popper, A.N. (1969)
- Mattheij, J.A. (1970)
- Mattheij, J.A. (1970)
- Elliott, W.R. (1970)
- Gertychowa, R. (1970)
- Gertychowa, R. (1970)
- Weiss, B. and Martini, J. (1970)
- Pfeiffer, W. (1970)
- Popper, A.N. (1970)
- Whitt, G.S. and Maeda, F.S. (1970)
- Wilkens, H. (1970)
- Wilkens, H. (1970)
- Wilkens, H. (1970)
- Wilkens, H. (1971)
- Reddell, J.R. and Mitchell, R.W. (1971)
- Gertychowa, R. (1971)
- Villalobos, A. (1971)
- Mollhagen, A. (1971)
- Popper, A.N. (1971)
- Wiley, S. and Mitchell, R.W. (1971)
- Wiley, S. and Mitchell, R.W. (1971)
- Zaccone, G. (1971)
- Wilkens, H. and Burns, R. J. (1972)
- Wilkens, H. (1972)
- Wilkens, H. (1972)
- Wilkens, H. (1972)
- Elliott, W.R. (1972)
- Parzefall, J. and Wilkens, H. (1972)
- Zaccone, G. (1972)
- Thines, G. and Wissocq, N. (1972)
- Avise, J.C. and Selander, R.K. (1972)
- Zeitlin, S. M. (1973)
- Voneida, T. J. (1973)
- Zeitlin, S. M. and McDevitt, D. S. (1973)
- Avise, J.C. and Kitto, G.B. (1973)
- Schemmel, C. (1973)
- Mitchell, R.W. (1973)
- Mitchell, R.W. and Elliott, W.R. (1973)
- Schmatolla, E. and Erdmann, G. (1973)
- Durand, J.P. (1973)
- Reddell, J.R. and Elliott, W.R. (1973)
- Yasuda, K. (1973)
- Peters, N. and Peters, G. (1973)
- Reddell, J.R. and Elliott, W.R. (1973)
- Mitchell, R.W. and Elliott, W.R. (1973)
- Wilkens, H. (1973)
- Peters, H. and Peters, G. (1973)
- Wilkens, H. (1973)
- Thines, G. and LeGrain, J. M. (1973)
- Schemmel, C. (1974)
- Schemmel, C. (1974)
- Egar, M.W. (1974)
- Pfeiffer, W. (1974)
- Sligar, C. (1974)
- Fish, J. (1974)
- Chakraborty, R. and Nei, M. (1974)
- Peters, N., Scholl, A. and Wilkens, H. (1975)
- Omura, Y. (1975)
- Pfeiffer, W. (1975)
- Sadoglu, P. (1975)
- Voneida, T. J. and Sligar, C. (1976)
- Wilkens, H. (1976)
- Durand, J.P. (1976)
- Wilkens, H (1976)
- Sligar, C. and Voneida, T.J. (1976)
- Herwig, H. J. (1976)
- Durand, J.P. (1976)
- Erckens, W. and Weber, F. (1976)
- Herwig, H. J. (1976)
- Cooper, J.E. (1977)
- Mitchell, R.W. and Russell, W.H. (1977)
- Fish, J. (1977)
- Wilkens, H. (1977)
- Edwards, R.J. (1977)
- Mitchell, R.W., Russell, W.H. and Elliott, W.R. (1977)
- Yew, D. T. and Yoshihara, H. M. (1977)
- Mitchell, R.W. and Elliott, W.R. (1977)
- Pfeiffer, W. (1977)
- Kirby, R.F., Thompson, K.W. and Hubbs, C. (1977)
- Mitchell, R.W. (1977)
- Mitchell, R.W. and Cooke, J.W. (1977)
- Mitchell, R.W. and Russell, W.H. (1977)
- Zaccone, G. (1977)
- Birkhead, W.S. (1978)
- Durand, J.P. (1978)
- Thines, G. and Weyers, M. (1978)
- Fish, S.E. and Voneida, T.J. (1979)
- Wilkens, H., Peters, H. and Schemmel, C. (1979)
- Woodhead, A. D. and Achey, P. M. (1979)
- Pactl, J. (1979)
- Durand, J.P. (1979)
- Weissert, R. (1979)
- Sadoglu, P. (1979)
- Wilkens, H. (1980)
- Schemmel, C. (1980)
- Wilkens, H. (1980)
- Weissert, R. (1980)
- Quinn, T. P. (1980)
- Campenhausen, C., Riess, I. and Weissert, R. (1981)
- Weissert, R. and Campenhausen, C. (1981)
- Erckens, W. (1981)
- Herwig, H. J. (1981)
- Erckens, W. (1981)
- Dolle, A. (1981)
- Reddell, J. R. (1981)
- Wilkens, H. (1981)
- Tabata, M. (1982)
- Romero, A. (1982)
- Jankowska, M. and Thines, G. (1982)
- Rose, F.L. and Mitchell, R.W. (1982)
- Erckens, W. and Martin, W. (1982)
- Erckens, W. and Martin, W. (1982)
- Lamprecht, G. and Weber, F. (1982)
- Senkel, S. (1983)
- Parzefall, J. (1983)
- Zilles, K., Tillmann, B. and Bennemann, R. (1983)
- Parzefall, J. (1983)
- Voneida, T.J., Fish, S.E. and Cauller, T.C. (1983)
- Romero, A. (1983)
- Parzefall, J. (1983)
- Romero, A. (1984)
- Voneida, T. J. and Fish, S. E. (1984)
- Romero, A. (1984)
- Fruhbeis, B. (1984)
- Mothes, P. and Jameson, R. (1984)
- Romero, A. (1984)
- Romero, A. (1984)
- Wilkens, H. (1984)
- Schuppa, M. (1984)
- Mangold-Wernado, U. and Pfeiffer, W. (1984)
- Hassan, E.S. (1985)
- Voneida, TJ and Fish, SE (1985)
- Huppop, K. (1985)
- Romero, A. (1985)
- Burchards, H., Dolle, A. and Parzefall, J. (1985)
- Fricke, D. (1985)
- Teyke, T. (1985)
- Romero, A. (1985)
- Romero, A. (1985)
- Wilkens, H. (1985)
- Burchards, H. and Parzefall, J. (1985)
- Wilkens, H. (1985)
- Lamprecht, G. and Weber, F. (1985)
- Hassan, E.S. (1985)
- Huppop, K. (1986)
- Wilkens, H. and Huppop, K. (1986)
- Parzefall, J. and Senkel, S. (1986)
- Parzefall, J. and Senkel, S. (1986)
- Parzefall, J. (1986)
- De Fraipont, M. and Thines, G. (1986)
- Huppop, K. (1986)
- De Fraipont, M. and Thines, G. (1986)
- Romero, A. (1986)
- Hassan, E.S. (1986)
- Wilkens, H. (1986)
- Hassan, E.S. (1986)
- Parzefall, J. (1986)
- De Fraipont, M. (1986)
- Fraipont, M. (1986)
- De Fraipont, M. (1987)
- De Fraipont, M. (1987)
- Huppop, K. (1987)
- Fricke, D. (1987)
- Wilkens, H. (1987)
- De Fraipont, M. (1988)
- Huppop, K. (1988)
- Teyke, T. (1988)
- Coombs, S., Janssen, J. and Webb, J. (1988)
- Fricke, D. (1988)
- Wilkens, H. (1988)
- Fack, H. and Wilkens, H. (1989)
- Fricke, D. and Parzefall, J. (1989)
- Morris, N (1989)
- Huppop, K. (1989)
- Teyke, T. (1989)
- Hassan, E.S. (1989)
- Huppop, K. (1989)
- Wilkens, H. (1989)
- Langecker, T. G. (1989)
- Parzefall, J. and Fricke, D. (1990)
- Peters, N., Schmidt, W. and Fricke, D. (1990)
- Langecker, T. G. (1990)
- Teyke, T. (1990)
- Peters, N. (1990)
- Langecker, T. G. (1990)
- Yokoyama, R. and Yokoyama, S. (1990)
- Yokoyama, R and Yokoyama, S (1990)
- Abdel-Latif, H., Hassan, E.S. and von Campenhausen, C. (1990)
- Klimpel, B. and Parzefall, J. (1990)
- Langecker, T. G. (1990)
- Parzefall, J. and Fricke, D. (1991)
- Riedel, G. and Krug, L. (1991)
- Huppop, K. and Wilkens, H. (1991)
- Langecker, T. G., Wilkens, H. and Junge, P. (1991)
- Bensouilah, M. and Denizot, J.P. (1991)
- Huppop, K. (1991)
- Campenhausen, C. (1991)
- Wilkens, H., Junge, P. and Langecker, T. G. (1991)
- Langecker, T.G. and Longley, G. (1992)
- Fish, S.E. and Ghosh, P. (1992)
- Wilkens, H. and Meyer, M. (1992)
- De Fraipont, M. (1992)
- Coombs S., Janssen J. and Montgomery J. (1992)
- Hassan, E. S., Abdel-Latif, H. and Biebricher, R. (1992)
- Langecker, T. G. (1992)
- Zafar, N.P. and Morgan, E. (1992)
- Lamprecht, G. and Weber, F. (1992)
- Langecker, T. G. (1992)
- Parzefall, J. (1992)
- Wilkens, H. (1992)
- Parzefall, J. (1992)
- Langecker, T. G. (1993)
- Yokoyama, R. and Yokoyama, S. (1993)
- Langecker, T. G., Schmale, H. and Wilkens, H. (1993)
- Peters, N., Schacht, V., Schmidt, W. and Wilkens, H. (1993)
- Langecker, T. G., Schmale, H. and Wilkens, H. (1993)
- Wilkens, H., Langecker, T. G. and Olcese, J. (1993)
- Wilkens, H. (1993)
- Hoffman, S. and Hausberg, C. (1993)
- Parzefall, J. (1993)
- Parzefall, J. (1993)
- Borowsky, R. (1994)
- Teyke, T. and Schaerer, S. (1994)
- Hausberg, C. (1994)
- Missall, J., Wilkens, H., Langecker, T. G. and Olcese, J. (1994)
- Missal, J, Wilkens, H, Langecker, TG and Olcese, 1994 (1994)
- Langecker, T. G., Neumann, B., Hausberg, C. and Parzefall, J. (1995)
- Yokoyama, R, Knox, BE and Yokoyama, S (1995)
- Langecker, T. G., Wilkens, H. and Schmale, H. (1995)
- Montgomery, J., Coombs, S. and Halstead, M. (1995)
- Yokoyama, S., Meany, A., Wilkens, H. and Yokoyama, R. (1995)
- Hausberg, C (1995)
- Wilkens, H. and Langecker, T. G. (1996)
- Jeffery, W.R., Hornaday, K. and Martasian, D.P. (1996)
- Borowsky, R. (1996)
- Riedel, G. (1997)
- Behrens, M., Langecker, T.G., Wilkens, H. and Schmale, H. (1997)
- Riedel, G. and Krug, L. (1997)
- Valdez-Moreno, M.F. (1997)
- Jeffery, W.R. and Martasian, D P. (1997)
- Borowsky, R. and Espinasa, L. (1997)
- Gamboa, VJ and Ku, L (1998)
- Wilkens, H. (1998)
- Behrens, M., Wilkens, H. and Schmale, H. (1998)
- Buckup, P.A. (1998)
- Contreras-Balderas, S. and Lozano-Vilano, M.D.L. (1998)
- Jeffery, W.R. and Martasian, D.P. (1998)
- Riedel, G. (1998)
- Wilkens, H. (1998)
- Baker, C.F. and Montgomery, J. (1999)
- Natke, C. (1999)
- Estrada, M. (1999)
- Kullander, S. O. (1999)
- Villalobos, J.L., Alvarez, F. and Iliffe, T.M. (1999)
- Pennisi, E. (2000)
- Yamamoto, Y. and Jeffery, W.R. (2000)
- Huppop, K. (2000)
- Ford, D.C. (2000)
- Jeffery, W.R., Strickler, A.G., Guiney, S., Heyser, D.G. and Tomarev, S.I. (2000)
- Espinasa, L. and Borowsky, R. (2000)
- Langecker, T.G. (2000)
- Strickler, A.G., Yamamoto, Y. and Jeffery, W.R. (2001)
- Avise, J.C. (2001)
- Jeffery, W.R. and Yamamoto, Y. (2001)
- Yokoyama, S, and Radlwimmer, F.B. (2001)
- Yamamoto, Y. and Jeffery, W.R. (2001)
- Parzefall, J and Hausberg, C (2001)
- Jeffery, W.R. (2001)
- Montgomery, J.C., Coombs, S. and Baker, C.F. (2001)
- Leclerc, P., Deharveng, L., Ng, P.K.L., Juberthie, C. and Decu, V. (2001)
- Boudriot, F and Reutter, K (2001)
- Espinasa, L., Rivas-Manzano, P. and Perez, H.E. (2001)
- Espinasa, L. and Borowsky, R. (2001)
- Espinasa, L. (2001)
- Krejca, J. (2002)
- Wilkens, H (2002)
- Berg, A. and Watson, G.M. (2002)
- Strickler, A.G., Famuditimi, K. and Jeffery, W.R. (2002)
- Dowling, T.E., Martasian, D.P. and Jeffery, W.R. (2002)
- Romero, A., Jeffery, W.R. and Yamamoto, Y. (2002)
- Coombs, S, New, JG and Nelson, M (2002)
- Parzefall, J and Hausberg, C (2002)
- Borowsky, RL and Wilkens, H (2002)
- Strecker, U. and Wilkens, H. (2002)
- Yamamoto, Y. and Jeffery, W.R. (2002)
- Carvalho, M.L., Oliveira, C., Navarrete, M.C., Froehlich, O. and Foresti, F. (2002)
- Sarma, S.S.S., Lopez-Romulo, A. and Nandini, S. (2003)
- Parry, J.W.L., Peirson, S. and Wilkens, H. (2003)
- Wilkens, H. and Strecker, U. (2003)
- Buckup, P.A. (2003)
- Romero, A., Green, S.M., Lelonek, M.M. and Stropnicky, K.C. (2003)
- Jeffery, W.R., Strickler, A.G. and Yamamoto, Y. (2003)
- Yamamoto, Y., Espinasa, L., Stock, D.W. and Jeffery, W.R. (2003)
- Reis, R.E., Kullander, S.O. and Ferraris, C.J. (2003)
- Valdez-Moreno, M. and Contreras-Balderas, S. (2003)
- Espinasa, L. and Jeffery, W.R. (2003)
- Espinasa, L. and Jeffery, W.R. (2003)
- Contreras-Balderas, S, Almada-Villela, P, Lozano-Vilano, M de L and Garcia-Ramirez, ME (2003)
- Strecker, U. (2003)
- Porter, M.L. and Crandall, K.A. (2003)
- Strecker, U., Bernatchez, L. and Wilkens, H. (2003)
- Angel, T.B. (2003)
- Wilkens, H (2004)
- McCauley, D.W., Hixon, E. and Jeffery, W.R. (2004)
- Yamamoto, Y (2004)
- Fuiman, L.A., Higgs, D.M. and Poling, K.R. (2004)
- Yamamoto, Y., Stock, D.W. and Jeffery, W.R. (2004)
- Hooven, T.A., Yamamoto, Y. and Jeffery, W.R. (2004)
- Peters, N (2004)
- Soares, D., Yamamoto, Y., Strickler, A.G. and Jeffery, W.R. (2004)
- Burt de Perera, T. (2004)
- Burt de Perera, T. (2004)
- Strecker, U., Faundez, V.H. and Wilkens, H. (2004)
- Moreira, C.R., Bichuette, M.E., Oyakawa, O.T., de Pinna, M.C.C. and Trajano, E. (2004)
- Jeffery, W.R. (2004)
- Fish, J. (2004)
- Burt de Perera, T and Braithwaite, VA (2005)
- Jeffery, W.R. (2005)
- Jeffery, W.R. (2005)
- Espinasa, L., Yamamoto, Y. and Jeffery, W.R. (2005)
- Panaram, K. and Borowsky, R.L. (2005)
- Kocher, T.D., Jeffery, W.R., Parichy, D.M., Peichel, C.L., Streelman, J.T. and Thorgaard, G.H. (2005)
- Tian, N.M.M.L. and Price, D.J. (2005)
- Trapani, J., Yamamoto, Y. amd Stock, D.W. (2005)
- Espinasa, L. and Espinasa, M. (2005)
- Espinasa, L. and Espinasa, M. (2005)
- Espinasa, L. and Jeffery, W.R. (2006)
- Jeffery, W.R. (2006)
- Protas, M.E., Hersey, C., Kochanek, D., Zhou, Y., Wilkens, H., Jeffery, W.R., Zon, L.I., Borowsky, R. and Tabin, C.J. (2006)
- Jeffery, W.R. (2006)
- Franz-Odendaal, T.A. and Hall, B.K. (2006)
- Plath, M., Rohde, M., Schroder, T., Taebel-Hellwig, A. and Schlupp, I. (2006)
- Reeves, R.G. and Bermingham, E. (2006)
- Peleshanko, S, Julian, MD, Ornatska, M, McConney, ME, LeMieux, MC, Chen, NN, Tucker, C, Yang, YC, Liu, C, Humphrey, JAC and Tsukruk, VV (2007)
- Di Palma, F., Kidd, C., Borowsky, R. and Kocher, T.D. (2007)
- Strickler, A.G., Byerly, M.S. and Jeffery, W.R. (2007)
- Kavalco, K.F. and Almeida-Toledo, L.F. (2007)
- Wilkens, H (2007)
- Porter, M.L., Dittmar, K. and Perez-Losada, M. (2007)
- Porter, M.L., Dittmar, K. and Perez-Losada, M (2007)
- Protas, M., Conrad, M., Gross, J.B., Tabin, C. and Borowsky, R.L. (2007)
- Holbrook, R., Bomphrey, R., Walker, S., Taylor, G., Thomas, A. and Burt de Perera, T. (2007)
- Menuet, A., Alunni, A., Joly, J.S., Jeffery, W.R. and Retaux, S. (2007)
- Alunni, A., Menuet, A., Candal, E., Penigault, J.B., Jeffery, W.R. and Retaux, S. (2007)
- Gregson, J.N.S. and Burt de Perera, T. (2007)
- Strickler, A.G., Yamamoto, Y. and Jeffery, W.R. (2007)
- Moreira, C.R. (2007)
- Borowsky, R.L. (2008)
- Borowsky, R.L. (2008)
- Windsor, S.P. (2008)
- Ornelas-Garcia, C.P., Dominguez-Dominguez, O. and Doadrio, I. (2008)
- Borowsky, R.L. (2008)
- Borowsky, R.L. (2008)
- Borowsky, R.L. (2008)
- Borowsky, R.L. (2008)
- Wilkens, H. (2008)
- Espinasa, M. and Espinasa, L. (2008)
- Borowsky, R. (2008)
- Yoshizawa, M. and Jeffery, W.R. (2008)
- Niven, J.E. (2008)
- Salin, K., Voituron, Y., Colson, C. and Hervant, F. (2008)
- Protas, M., Tabansky, I., Conrad, M., Gross, J.B., Vidal, O., Tabin, C. and Borowsky, R.L. (2008)
- Yong, E. (2008)
- Holbrook, R., Bomphrey, R., Walker, S., Taylor, G., Thomas, A. and Burt de Perera, T. (2008)
- Gross, J.B., Protas, M., Conrad, M., Scheid, P.E., Vidal, O., Jeffery, W.R., Tabin, C. and others (2008)
- Holbrook, R.I. and Burt de Perera, T. (2008)
- Retaux, S, Pottin, K and Alunni, A (2008)
- Aw, J.M., Holbrook, R.I., Burt de Perera, T. and Kacelnik, A. (2008)
- Windsor, S., Mallinson, G. and Montgomery, J. (2008)
- Strecker, U. (2008)
- Windsor, S., Tan, D. and Montgomery, J. (2008)
- Jeffery, W.R. (2008)
- Moreira, C.R. and Trajano, E. (2008)
- Jeffery, W.R. (2008)
- Holbrook, R. and Burt de Perera, T. (2009)
- Albertson, R.C., Cresko, W., Detrich, H.W. and Postlethwait, J.H. (2009)
- Patton, P., Coombs, S. and Windsor, S. (2009)
- Jeffery, W.R. (2009)
- Jeffery, W.R. (2009)
- Maher, B. (2009)
- Streets, A and Soares, D (2009)
- Porter, M.L., Jeffery, W.R. and Dittmar, K. (2009)
- Jeffery, W.R. (2009)
- Esquivel Bobadilla, S., Borowsky, R.L., Espinosa Perez, H. and Garcia de Leon, F.J. (2009)
- Borowsky, R.L. (2009)
- Jeffery, W.R. (2009)
- McHenry, M., Feitl, K. and Cardenas, G. (2009)
- Culver, D.C. and Pipan, T. (2009)
- Reynoso, V.H., Paredes-Leon, R. and Arroyo Lambaer, D. (2009)
- Ornelas-Garcia, C.P., Dominguez-Dominguez, O. and Doadrio, I. (2009)
- Strickler, A.G. and Jeffery, W.R. (2009)
- Yoshizawa, M., Soares, D., Goricki, S., Ashida, G. and Jeffery, W.R. (2009)
- De Carvalho, P.A., Paschoalini, A.L., Santos, G.B., Rizzo, E. and Bazzoli, N. (2009)
- Cronk, Q.C.B. (2009)
- Yamamoto, Y., Byerly, M.S., Jackman, W.R. and Jeffery, W.R. (2009)
- Varatharasan, N., Croll, R. and Franz-Odendaal, T. (2009)
- Valdez-Moreno, M., Ivanova, N.V., Elias-Gutierrez, M., Contreras-Balderas, S. and Herbert, P.D.N. (2009)
- Sharma, S., Coombs, S., Patton, P. and Burt de Perera, T. (2009)
- Franz-Odendaal, T., Varatharasan, N. and Croll, R.P. (2009)
- Leclercq, E., Taylor, J.F. and Migaud, H. (2009)
- McConney, M.E., Chen, N.N., Lu, D., Hu, H., Coombs, S., Liu, C. and Tsukruk, V.V. (2009)
- Coombs, S. and Patton, P. (2009)
- Kowalko, J., Gross, J.B., Protas, M., Borowsky, R.L. and Tabin, C. (2009)
- Dufton, M. and Franz-Odendaal, T.A. (2009)
- Ren, W.Y. and Yamamoto, Y. (2009)
- Pottin, K and Retaux, S (2009)
- Gross, J.B., Borowsky, R. and Tabin, C. (2009)
- Sutherland, L., Holbrook, R.I. and Burt de Perera, T. (2009)
- Guibal, C., Yamamoto, Y., Reynoso, V.H. and Whitmore, D. (2009)
- Retaux, S., Alunni, A., Menuet, A. and Pottin, K. (2009)
- Aw, J.M., Holbrook, R.I., Burt de Perera, T. and Kacelnik, A. (2009)
- Espinasa, L. and Giribet, G. (2009)
- Whitmore, D., Guibal, C., Tamai, K. and Yamamoto, Y. (2009)
- Windsor, S. (2009)
- Holbrook, R. and Burt de Perera, T. (2009)
- Jeffery, W.R. (2010)
- Moreira, C.R., Bichuette, M.E., Oyakawa, O.T., de Pinna, M.C.C. and Trajano, E. (2010)
- Taylor, G.K., Holbrook, R. and Burt de Perera, T. (2010)
- Patton, P., Windsor, S. and Coombs, S. (2010)
- Windsor, S.P., Norris, S.E., Cameron, S.M., Mallinson, G.D. and Montgomery, J.C. (2010)
- Mirande, J.M. (2010)
- Van Trump, W.J., Coombs, S., Duncan, K. and McHenry, M. (2010)
- Windsor, S.P., Norris, S.E., Cameron, S.M., Mallinson, G.D. and Montgomery, J.C. (2010)
- Jeffery, W.R. (2010)
- Juan, C., Guzik, M.T., Jaume, D. and Cooper, S.J.B. (2010)
- Jeffery, W.R. and Strickler, A.G. (2010)
- Wilkens, H (2010)
- Yoshizawa, M., Goricki, S., Soares, D. and Jeffery, W.R. (2010)
- Pottin, K., Hyacinthe, C., and Retaux, S. (2010)
- Salin, K., Voituron, Y., Mourin, J. and Hervant, F. (2010)
- Coombs. S. (2010)
- Gross, J.B. and Tabin, C.J. (2010)
- Van Trump, W.J., Coombs, S., Duncan, K. and McHenry, M.J. (2010)
- Retaux, S, Pottin, K and Hyacinthe, C (2011)
- Jeffery, W.R. and Bilandzija, H. (2011)
- Espinasa, L. (2011)
- Retaux, S (2011)
- Holbrook, R. and Burt de Perera, T. (2011)
- Pottin, K., Hinaux, H. and Retaux, S. (2011)
- Kish, P.E., Bohnsack, B.L., Gallina, D., Kasprick, D.S. and Kahana, A. (2011)
- Gatenby, R.A., Gillies, R.J. and Brown, J.S. (2011)
- Holbrook, R. and Burt de Perera, T. (2011)
- Kasumyan, A.O. (2011)
- Windsor, S., Paris, J. and Burt de Perera, T. (2011)
- Ornelas-Garcia, C.P., Pedraza-Lara, C.P., Bastir, M. and Doadrio, I. (2011)
- Ma, L and Jeffery, WR (2011)
- Avise, J.C. (2011)
- Wilkens, H. (2011)
- Gallo, N.D. and Jeffery, W.R. (2011)
- Duboue, E.R. and Borowsky, R.L. (2011)
- Duboue, E.R., Keene, A. and Borowsky, R. (2011)
- Strickler, A.G. and Soares, D. (2011)
- Vazquez-Echeverria, C., Guibal, C., Reynoso, V.H., Carrillo-Rosas, S., Ramos-Balderas, J.L. and Maldonado, E. (2011)
- Yoshizawa M. and Jeffery, W.R. (2011)
- Yamamoto, Y. and Jeffery, W.R. (2011)
- Kowalko, J., Rohner, N., Borowsky, R.L. and Tabin, C. (2011)
- Lechner, W. and Ladich, F. (2011)
- Yoshizawa, M. and Jeffery, W.R. (2011)
- Salin, K., Voituron, Y., Mourin, J. and Hervant, F. (2011)
- Elipot, Y., Callebert, J., Hinaux, H. and Retaux, S. (2011)
- Cavallari, N., Frigato, E., Vallone, D., Froehlich, N., Lopez-Olmeda, J.F., Foa, A., Berti, R., Sanchez-Vazquez, F.J., Bertolucci, C. and Foulkes, N.S. (2011)
- Oliveira, C., Avelino, G.S., Abe, K.T., Mariguela, T.C., Benine, R.C., Orti, G., Vari, R.P. and Correa e Castro, R.M. (2011)
- Pottin, K., Hinaux, H., Elipot, Y., Chaloub, H., Pere, S. and Retaux, S. (2011)
- Bradic, M., Beerli, P. and Borowsky, R.L. (2011)
- Tan, D., Patton, P. and Coombs, S. (2011)
- Legendre, L., Elipot, Y., Hinaux, H., Pere, S., Sohm, F. and Retaux, S. (2011)
- Schobert, C.S., Stiassny, M.L.J., Jeffery, W.D. and Dubielzig, R.R. (2011)
- Hinaux, H., Pottin, K., Chalhoub, H., Pere, S., Elipot, Y., Legendre, L. and Retaux, S. (2011)
- Hausdorf, B., Wilkens, H. and Strecker, U. (2011)
- Esquivel-Bobadilla, S (2011)
- Hinaux, H and Retaux, S (2011)
- Hinaux, H., Fartman, B., DaSilva, C., Noirot, C. and Jeffery, W.R. (2011)
- Strecker, U., Hausdorf, B. and Wilkens, H. (2012)
- Yoshizawa, M., Yamamoto, Y., O'Quin, K.E. and Jeffery, W.R. (2012)
- Dufton, M., Hall, B.K. and Franz-Odendaal, T. (2012)
- Porter, M.L., Blasic, J.R., Bok, M.J., Cameron, E.G., Pringle, T., Cronin, T.W. and Robinson, P.R. (2012)
- Jeffery, W.R. (2012)
- Pipan, T. and Culver, D.C. (2012)
- Huppop, K. (2012)
- Gallo, N.D. and Jeffery, W.R. (2012)
- Yoshizawa, M., Ashida, G. and Jeffery, W.R. (2012)
- Hoke, K., Schwartz, A. and Soares, D. (2012)
- Idda, M.L., Bertolucci, C., Vallone, D., Gothilf, Y., Sánchez-Vázquez, F.J. and Foulkes N.S. (2012)
- Gross, J. B. and Wilkens, H. (2012)
- Duboue, E.R., Borowsky, R.L. and Keene, A.C. (2012)
- Duboue, E.R. and Borowsky, R. (2012)
- Wilkens, H. (2012)
- Mogdans, J. and Bleckmann, H. (2012)
- Trontelj, P. (2012)
- Hervant, F. (2012)
- Gross, J.B. (2012)
- Protas, M. and Jeffery, W.R. (2012)
- Franck, D (2012)
- Bradic, M., Beerli, P., Garcia-de Leon, F.J., Esquivel-Bobadilla, S. and Borowsky, R.L. (2012)
- Blin, M., Bibliowicz, Y. and Rétaux, S. (2013)
- Stahl, A.L., Stahl, B.A., Buschbeck, E. and Gross, J.B. (2013)
- Hinaux, H., Poulain, J., Da Silva, C., Noirot, C., Jeffery, W. R., Casane, D. and Retaux, S. (2013)
- OʼQuin, K.E., Yoshizawa, M., Doshi, P. and Jeffery, W.R. (2013)
- Wall, A. and Volkoff, H. (2013)
- Bibliowicz, J., Elipot, Y., Blin, M. and Rétaux, S. (2013)
- Bilandzija, H., Ma, L., Parkhurst, A. and Jeffery, W.R. (2013)
- Kowalko, J.E., Rohner, N., Rompani, S.B., Peterson, B.K., Linden, T.A., Yoshizawa, M., Kay, E.H., Weber, J., Hoekstra, H.E., Jeffery, W.R., Borowsky, R. and Tabin, C.J.. (2013)
- Coghill, L.M. (2013)
- Friedrich, M. (2013)
- Espinasa, L. (2013)
- Pennisi, E. (2013)
- Borowsky, R. and Cohen, D. (2013)
- Espinasa, L. and Jeffery, W. (2013)
- Atukorala, A.D.S., Hammer, C., Dufton, M. and Franz-Odendaal, T.A. (2013)
- Bobadilla, S.E., García de León, F.J. and Borowsky, R. (2013)
- Retaux, S. and Casane, D. (2013)
- Van Trump, W.J. and McHenry, M.J. (2013)
- Borowsky, R. (2013)
- Retaux, S., Elipot, Y., Prunier, L. Hinaux, H. and Blin, M. (2013)
- Borowsky, R. (2013)
- Vazquez, A.O.S., Bárcenas-Luna, R. and Arellano-Carbajal, F. (2013)
- Gunter, H. and Meyer, A. (2013)
- Retaux, S. and Elipot, Y. (2013)
- Beale, A., Guibal, C., Tamai, T.K., Klotz, L., Cowen, S., Peyric, E., Reynoso, V.H., Yamamoto, Y. and Whitmore, D. (2013)
- Stemmer, M., Schuhmacher, L.-N., Foulkes, N.S., Bertolucci, C. and Wittbrodt, J. (2013)
- Garcia-Gonzalez, O.M. and Arellano-Carbajal, F. (2013)
- Beale, A. (2013)
- Ma, L. and Jeffery, W. (2013)
- Rétaux, S., Bourrat, F., Joly, J.-S. and Hinaux, H., (2013)
- Rodrigues, F.R. (2013)
- Gross, J. B., Krutzler, A.J. and Espinasa, L. (2013)
- Pazza, R., Bertollo, L.A.C., de Almeida-Toledo, L.F. and Kavalco, K.F. (2013)
- Jeffery, W., Doshi, P., Yoshizawa, M. and O’Quin, K.E. (2013)
- Gross, J.B., Krutzler, A.J. and Bruns, L.E. (2013)
- Joachim, B.L., Riesch R., and Jeffery, W.R., and Schlupp, I. (2013)
- Gross, J.B. and Wilkens, H. (2013)
- Gross, J. B. and Matthews, M (2013)
- Elliott, W.R. (2013)
- Borowsky, R.L. (2013)
- Hinaux, H., Poulain, J., Da Silva, C., Noirot, C., Jeffery, W.R., Casane, D. and Rétaux, S. (2013)
- Kowalko, J.E., Rohner, N., Rompani, S.B., Peterson, B.K., Linden, T.A., Yoshizawa, M., Kay, E.H., Weber, J., Hoekstra, H.E., Jeffery, W.R., Borowsky, R. and Tabin, C.J. (2013)
- Keene, A. (2013)
- O'Quin, K.E., Yoshizawa, M., Doshi, P. and Jeffery, W R. (2013)
- Bibliowicz, J., Alie, A., Espinasa, L., Yoshizawa, M., Blin, M., Hinaux, H., Legendre, L., Pere, S. and Retaux, S. (2013)
- Krutzler, A.J., Bruns, L.E. and Gross, J.B. (2013)
- Rohner, N., Jarosz, D.F., Kowalko, J.E., Yoshizawa, M., Jeffery, W.R., Borowsky, R.L., Lindquist, S. and Tabin, C.J. (2013)
- Yoshizawa, M., O'Quin, K.E. and Jeffery, W.R. (2013)
- Gross, J.B., Furterer, A., Carlson, B.M. and Stahl, B.A. (2013)
- Legendre, L., Elipot, Y., Hinaux, H., Pere, S., Sohm, F. and Retaux, S. (2013)
- Furterer, A., Carlson, B.M., Stahl, B.A. and Gross, J.B. (2013)
- Rohner, N., Jarosz, D.F., Taipale, M., Kowalko, J., Yoshizawa, M., Jeffery, W.R., Borowsky, R.L., Lindquist, S. and Tabin, C.J. (2013)
- Yoshizawa, M., O'Quin, K.E., Ashida, G. and Jeffery, W.R. (2013)
- Elipot, Y., Hinaux, H., Callebert, J. and Retaux, S. (2013)
- Bradic, M., Teotonio, H. and Borowsky, R.L. (2013)
- Stahl, B.A. and Gross, J.B. (2013)
- Kowalko, J., Rohner, N., Linden, T.A., Rompani S.B., Warren, W.C., Borowsky, R., Tabin, C.J., Jeffery, W.R. and Yoshizawa M. (2013)
- Yoshizawa, M., O’Quin, K.E., and Jeffery, W.R. (2013)
- Yoshizawa, M., Jeffery, W.R., van Netten, S.M. and McHenry, M.J. (2014)
- Elipot, Y., Hinaux, H., Callebert, J., Launay, J.-M., Blin, M. and Retaux, S. (2014)
- Bleckmann, H., Mogdans, J. and Coombs, S.L. (2014)
- Holzman, R., Perkol-Finkel, S. and Zilman, G. (2014)
- Trajano, E. and Moreira, C.R. (2014)
- Espinasa, L., Bibliowicz, J., Jeffery, W. R. and Retaux, S. (2014)
- Atukorallaya, D. and Franz-Odendaal, T. (2014)
- Espinasa, L., Bartolo, N.D. and Newkirk, C.E. (2014)
- Espinasa, L., Centone, D.M. and Gross, J.B. (2014)
- Atukorala, A.D.S. and Franz-Odendaal, T.A. (2014)
- McGaugh, S.E., Gross, J.B., Aken, B., Blin, M., Borowsky, R., Chalopin, D., Hinaux, H., Jeffery, W.R., Keene, A., Ma, L., Minx, P., Murphy, D., O'Quin, K.E., Retaux, S., Rohner, N., Searl, S.M., Stahl, B.A., Tabin, C., Volff, J.N., Yoshizawa M, Warren WC. (2014)
- Moran, D., Softley, R. and Warrant, E.J. (2014)
- Windsor, S.P. (2014)
- Penney, C.C. and Volkoff, H. (2014)
- Ma, L., Parkhurst, A. and Jeffery, W.R. (2014)
- Elliott, W.R. (2014)
- Coghill, L.M., Hulsey, C.D., Chaves-Campos, J., de Leon, F.J.G. and Johnson, S.G. (2014)
- Soares, D. Niemiller, M.L. and Higgs, D. (2014)
- Schartl, M. (2014)
- Montgomery, J., Bleckmann, H. and Coombs, S. (2014)
- Gross, J.B., Krutzler, A.J. and Carlson, B.M. (2014)
- Elipot, Y., Legendre, L., Pere, S., Sohm, F. and Retaux, S. (2014)
- Coombs, S., Bleckmann, H., Fay, R.R. and Popper, A.N. (2014)
- Palacios-Vargas, J.G., Juberthie, C. and Reddell, J. (2014-2015)
- Caballero-Hernandez, O., Hernandez-Patricio, M., Sigala-Regalado, I., Morales-Malacara, J.B. and Miranda-Anaya, M. (2015)
- Yoshizawa, M. and Macaspac, C. (2015)
- Riddle, M. and Tobin, C.J. (2015)
- Stahl, B.A. and Gross, J.B. (2015)
- Kulpa, M., Bak-Coleman, J. and Coombs, S. (2015)
- O’Quin, K.E., Doshi, P., Lyon, A., Hoenemeyer, E., Yoshizawa, M. and Jeffery, W,R. (2015)
- Bradic, M, Rohner, N, Tabin, C and Borowsky, R. (2015)
- Yoshizawa, M. (2015)
- Stahl, B.A., Ma, L. and Gross, J.B. (2015)
- Reyes, W.D. (2015)
- Bilandzija, H. (2015)
- Rohner, N., Aspiras, A., Borowsky, R. and Tabin, C. (2015)
- Soares, D. (2015)
- Kasumyan, A. O. and Marusov, E. A. (2015)
- Powers, A.K., Sung, J.Y.T. and Gross, J.B. (2015)
- Keene, A. (2015)
- Silva, D. M. Z. A., Utsunomia, R., Pansonato-Alves, J.C., Oliveira, C. and Foresti, F. (2015)
- Aspiras, A.C., Rohner, N., Martineau, B., Borowsky, R.L. and Tabin, C.J. (2015)
- Robinson, B.G., Jaggard, J.B., Yoshizawa, M. and Keene, A.C. (2015)
- Dufton, M. and Franz-Odendaal, T.A. (2015)
- McGaugh, S. E., Weagley, J., Jeffery, W.R., Yoshizawa, M., O'Quin, K., Espinasa, L., Rohner, N., Borowsky, R. and Warren, W. (2015)
- Moran, D., Softley, R. and Warrant, E.J. (2015)
- Aspiras A.C., Rohner N., Martineau B., Borowsky R.L. and Tabin C.J. (2015)
- Hinaux, H., Blin, M., Fumey, J., Legendre, L., Heuze, A., Casane, D. and Retaux, S. (2015)
- Yoshizawa, M., Robinson, B.G., Duboue, E.R., Masek, P., Jaggard, J.B., O'Quin, K.E., Borowsky, R.L., Jeffery, W.R. and Keene, A.C. (2015)
- Hinaux, H., Recher, G., Blin, M., Devos, L., Alié, A. and Rétaux, S. (2015)
- Stemmer, M., Schuhmacher, L.-N., Foulkes, N.S., Bertolucci, C. and Wittbrodt, J. (2015)
- Fumey, J., Hinaux, H., Noirot, C., Rétaux S. and Casane, D. (2015)
- Hernandez Mena, D., Santacruz Vázquez, A.O., Ornelas García, P., Mendoza Garfias, B., Ponce de León, G.P. (2015)
- Jaggard, J.B., Robinson, B., Oh, I., Masek, P., Yoshizawa, M. and Keene, A. (2015)
- Ma, L., Carlson, B., Stahl, B., Powers, M. and Gross, J.B. (2015)
- Niven, J.E. (2015)
- Ma, L., Jeffery, W.R., Essner, J.J. and Kowalko, J.E. (2015)
- Hammer, C., Atukorala, A.D.S. and Franz‐Odendaal, T. (2015)
- Tabin, J., Rohner, N., Haro, A., Kowalko, J., Martineau, B., Borowsky, R. and Tabin, C. (2015)
- Ma, L. and Jeffery, W.R. (2015)
- Gross, J.B., Meyer, B. and Perkins, M. (2015)
- Devos, L, Alie, A. and Retaux, S. (2015)
- Sumi, K., Asaoka, R., Nakae, M. and Sasaki, K. (2015)
- Garcia, O.M., Santacruz, A., Reynoso, V.H. and Maldonado, E. (2015)
- Carlson, B.M., Onusko, S.W. and Gross, J.B. (2015)
- Carlson, B.M. and Gross, J.B. (2016)
- Hinaux, H., Retaux, S. and Elipot, Y. (2016)
- Yamamoto, Y. (2016)
- Volkoff, H. (2016)
- Hinaux, H., Devos, L., Blin, M., Elipot, Y., Bibliowicz, J., Alei, A. and Retaux, S. (2016)
- Kowalko, J.E., Ma, L. and Jeffery, W.R. (2016)
- Gross, J.B. (2016)
- Ma, L., Stahl, B., Adams, H. and Gross, J. (2016)
- Yoshizawa, M. (2016)
- Pennisi, E. (2016)
- Gross, J.B., Stahl, B.A., Powers, A.K. and Carlson, B.M. (2016)
- Jeffery, W.R., Ma, L., Parkhurst, A. and Bilandzija, H. (2016)
- Rohner, N. (2016)
- Burt de Perera, T., Holbrook, R. and Davis, V. (2016)
- Pennisi, E (2016)
- Borowsky, R. (2016)
- Bilandžija, H. (2016)
- Casane, D. and Retaux, S. (2016)
- Gross, J.B. and Powers, A.K. (2016)
- Yoshizawa, M., Settle, A., Macaspac, C., Fernandes, V. Yoshida, M. and Keene, A. (2016)
- Aspiras, A., Tabin, C. and Rohner, N. (2016)
- Bilandžija, H. (2016)
- Gross, J.B., Gangidine, A and Powers, A.K. (2016)
- Espinasa, L. and Espinasa, M. (2016)
- Shoen, E., Jeffery, W. and Bilandžija, H. (2016)
- Gross, J.B., Powers, A.K., Davis, E.M. and Kaplan, S.A. (2016)
- Powers, A. Davis, E., Kaplan, S. and Gross, J. (2016)
- Atukorala, A.D.S. and Franz-Odendaal, T.A. (2016)
- Beale, A.D. and Whitmore, D. (2016)
- Hollifield, B., Bilandžija, H. and Jeffery, W. (2016)
- O'Quin, K. and McGaugh, S.E. (2016)
- Beale, A., Whitmore, D. and Moran, D. (2016)
- Schmitter-Soto, J.J. (2016)
- Martin, L., Bilandžija, H., Soueidan, S. and Jeffery, W. (2016)
- Foulkes, N.S., Whitmore, D., Vallone, D and Bertolucci, C. (2016)
- Keene, A.C., Yoshizawa, M. and McGaugh, S.E. (2016)
- Piscor, D. and Parise-Maltempi, P. P. (2016)
- Wilkens, H (2016)
- Soares, D., Niemiller, M.L. and Higgs, D.M. (2016)
- Santacruz, A., Garcia, O.M.., Tinoco-Cuellar, M., Rangel-Huerta, E. and Maldonado, E. (2016)
- Duboue, E.R. and Keene, A.C. (2016)
- Piscor, D., Centofante, L and Parise-Maltempi, P. P. (2016)
- Elliott, W.R. (2016)
- Cartwright, R.A., Schwartz, R.S., Merry, A.L. and Howell, M.M. (2016)
- Rossini, B.C., Oliveira, C.A.M., Melo, F.A.G., Bertaco, V.A., Astarloa, J.M.D., Rosso, J.J., Fauto, F. and Oliveira, C. (2016)
- Lyon, A., Krutzler, A.J., Gross, J.B. and O'Quin, K.E. (2016)
- Elliott, W.R. (2016)
- Tabin, C.J. (2016)
- Jeffery, W.R. (2016)
- O'Quin, K.E., Doshi, P., Lyon, A., Hoenemeyer, E., Yoshizawa, M. and Jeffery, W.R. (2016)
- Jeffery, W.R. (2016)
- Jeffery, W.R. (2016)
- Retaux, S., Alie, A., Blin, M., Devos, L., Elipot, Y. and Hinaux, H. (2016)
- Gorički, S., Stanković, D., Snoj, A., Kuntner, M., Jeffery, W.R., Trontelj, P., Pavićević, M., Grizelj, Z., Năpăruş-Aljančič, M. and Aljančič, G. (2017)
- Cartwright, R.A., Schwartz, R.C., Merry, A.L. and Howell, M.M. (2017)
- Retaux, S., Bibliowicz, J., Blin, M., Elipot, Y., Espinasa, L., Hinaux, H. and Tine, E. (2017)
- Mifsud. N. and Baker, N. (2017)
- Riddle, M., Finley, M., Marancik, D., Martineau, B. and Tabin, C. (2017)
- Jaggard, J.B., Robinson, B.G., Stahl, B, Oh, I., Masek, P., Yoshizawa, M. and Keene, A. (2017)
- Krishnan, J., Seidel, C. and Rohner, N. (2017)
- Krishnan, J. and Rohner, N. (2017)
- Ornelas-Garcia, C.P., Powers, A., Berning, D., Rodiles, R., Barluenga, M. and Gross, J.B. (2017)
- Tuccinardi, M.J. (2017)
- Stahl, B.A., Jaggard, J.B., Duboue, E.R. and Keene, A.C. (2017)
- Fernandes, V., Macaspac, C. and Yoshizawa, M. (2017)
- Hyacinthe, C., Aattia, J., Froc, C. and Retaux, S. (2017)
- Yoshizawa, M., Valdez, C., Yew, J., Coyle, K. and Roberts, R. (2017)
- Collins, E., Rutkowski, J., Ornelas García, P., Rétaux, S., Rohner, N. and Espinasa, L. (2017)
- Riddle, M.R.; Tabin, C.J (2017)
- Stahl, B.A. and Gross, J.B. (2017)
- Aspiras, A.C., Riddle, M., Zueckert-Gaudenz, K., Peuß, R., Martineau, B., Box, A., Levy, M., Tabin, J.A., McGaugh, S., Borowsky, R., Tabin, C.J. and Rohner, N. (2017)
- Espinasa, L., Bonaroti, N., Wong, J., Pottin, K., Queinnec, E. and Retaux, S. (2017)
- Kopp, J., Coppola, J. and Espinasa, L. (2017)
- Alvarado, C.G., Gálvez Hernández, O., Garduño Sánchez, M. and Ornelas García, C.P. (2017)
- Powers, A.K., Davis, E.M., Kaplan, S.A. and Gross, J.B. (2017)
- Espinasa, L., Gibbon, D., Rutkowski, J., Collins, E. and Rohner, N. (2017)
- Bonaroti, N., Rutkowski, J., Rétaux, S., Collins, E., Ornelas García, P., Heintz, C. and Espinasa, L. (2017)
- Poulson, T.L (2017)
- Box, A.C., Peuß, R., Haug, J. and Rohner, N. (2017)
- Powers, A.K., Davis, E.M., Kaplan, S.A. and Gross, J.B. (2017)
- Schmitter-Soto, J.J. (2017)
- Delić, T., Trontelj, P., Rendoš, M. and Fišer, C. (2017)
- Herman, A., Weagley, J., Jeffery, W.R., Borowsky, R., Brandvain, Y., Bilandžija, H., Espinasa, L., O'Quin, K., Ornelas-García, C., Gilbertson, E., Passow, C., Yoshizawa, M., Warren, W. and McGaugh, S.E. (2017)
- Fumey, J., Hinaux, H., Noirot, C., Thermes, C., Retaux, S. and Casane, D. (2017)
- Peña-Herrejón, Guillermo A. Sanchez-Velazquez, Julieta Cruz-Hernández, Andrez Aguirre-Becerra, Humberto García-Trejo, Fernando (2017)
- Jaggard, J.B., Robinson, B.G., Stahl, B.A., Oh, I., Masek, P., Yoshizawa, M. and Keene, A.C. (2017)
- Wilkens, H. and Strecker, U. (2017)
- Lyon, A., Powers, A.K., Gross, J.B. and O'Quin, K.E. (2017)
- Torres-Paz, J., Alié, A., Devos, L., Prunier, L., Boulet, F., Blin, M., Elipot, Y. and Rétaux, S. (2017)
- Maldonado, E., García O.M. and Santacruz, A. (2017)
- Gross, J.B. (2017)
- Gore, A.V., Tomins, K., Iben, J., Castranova, D., Davis, A., Parkhurst, A., Soueidan, S., Jeffery, W.R. and Weinstein, B.M. (2017)
- Pierre, C., Froc, C., Callebert, J. and Rétaux, S. (2017)
- Riddle, M., Aspiras, A., Tabin, J., Damen, F., Martineu, B., Peavey, M. and Tabin, C. (2017)
- Di Tizio, L., Di Francesco, N., Pennelli, M. and Placentile, G. (2017)
- Simon, N., Fujita, S., Porter, M. and Yoshizawa, M. (2017)
- Gore, A.V., Tomins, K.A., Iben, J., Ma, L., Castranova, D., Davis, A.E., Parkhurst, A., Jeffery, W.R. and Weinstein, B.M. (2017)
- Tabin, J., Rohner, N., Haro, A., Kowalko, J., Martineau, B., Borowsky, R. and Tabin, C. (2017)
- Kaplan, S.A., Powers, A.K. and Gross, J.B. (2017)
- Simon, V., Elleboode, R., Mahé,K. Legendre, L., Ornelas‑Garcia, P., Espinasa, L. and Rétaux, S. (2017)
- Jeffery, W.R., Ma, L., Tomins, K., Castranova, D., Weinstein, B.M. and Gore, A. (2017)
- Hinaux, H., Recher, G., Alie, A., Legendre, L., Blin, M. and Retaux, S. (2017)
- Ma, L., Strickler, A.G., Parkhurst, A., Yoshizawa, M., Shi, J. and Jeffery, W.R. (2018)
- Alie, A., Devos, L., Torres-Paz, J., Prunier, L., Boulet, F., Blin, M., Elipot, Y. and Retaux, S. (2018)
- Tang, J.L.Y., Guo, Y., Stockdale, W.T., Rana, K., Killen, A.C., Mommersteeg, M.T.M. and Yamamoto, Y. (2018)
- Atukorallaya, D.S.A. and Ratnayake, R.K. (2018)
- Xiong, S., Krishnan, J., Peuß, F. and Rohner, N. (2018)
- Carlson, B.M. and Gross, J.B. (2018)
- Ren, X.Y., Hamilton, N., Müller, F. and Yamamoto, Y. (2018)
- Stockdale, W.T., Lemieux, M.E., Killen, A.C., Zhao, J., Hu, J., Riepsaame, J. Hamilton, N., Kudoh, T., Riley, P.R., van Aerle, R., Yamamoto, Y. and Mommersteeg, M.T.M. (2018)
- Espinasa, L. (2018)
- Carlson, B.M., Klingler, I.B., Meyer, B.J. and Gross, J.B. (2018)
- Jeffery, W.R. (2018)
- Sears, C.R. and Gross, J.B. (2018)
- Elliott, W.R. (2018)
- Espinasa, L., Legendre, L., Fumey, J., Blin, M., Rétaux, S. and Espinasa, M. (2018)
- Davis, V.A., Holbrook, R.I. and Burt de Perera, T. (2018)
- Torres-Dowdall, J., Karagic, N., Plath, M. and Riesch, R. (2018)
- Devitsina, G.V. and Golovkina, T.V. (2018)
- Mirande, J.M. (2018)
- Klaassen, H., Wang, Y., Adamski, K., Rohner, N. and Kowalko, J.E. (2018)
- Koch, L. (2018)
- Powers, A.K., Boggs, T.E. and Gross, J.B. (2018)
- Borowsky, R. (2018)
- Rohner, N. (2018)
- Tabin, J.A., Aspiras, A., Martineau, B., Riddle, M., Kowalko, J., Borowsky, R., Rohner, N. and Tabin, C.J. (2018)
- Riddle, M.R., Aspiras, A.C., Gaudenz, K., Peuß, R., Sung, J.Y., Martineau, B., Peavey, M., Box, A.C., Tabin, J.A., McGaugh, S., Borowsky, R., Tabin, C.J. and Rohner, N. (2018)
- Borowsky, R., Luk, A., He, X.J. and Kim, R.S. (2018)
- Ornelas-García, P., Pajares, S., Sosa-Jiménez, V.M., Rétaux, S. and Miranda-Gamboa, R.A. (2018)
- Pagano, C., Siauciunaite, R., Idda, M.L., Ruggiero, G., Ceinos, R.M., Pagano, M., Frigato, E., Bertolucci, C., Foulkes, N.S. and Vallone, D. (2018)
- Yoshizawa, M., Settle, A., Hermosura, M.C., Tuttle, L.J., Cetraro, N., Passow, C.N. and McGaugh, S.E. (2018)
- Fernandes, V.F.L., Macaspac, C., Lu, L. and Yoshizawa, M. (2018)
- Rohner, N. (2018)
- Fumey, J., Hinaux, H., Noirot, C., Thermes, C., Rétaux, S. and Casane, D. (2018)
- Riddle, M.R., Boesmans, W., Caballero, O, Kazwiny, Y. and Tabin,C.J. (2018)
- Atukorala, A.D.S. and Franz-Odendaal, T.A. (2018)
- Garita-Alvarado, C.A., Barluenga, M. and Ornelas-García, C.P. (2018)
- Frøland Steindal, I.V., Beale, A.D., Yamamoto, Y. and Whitmore, D. (2018)
- Gross, J.B., Weagley, J., Stahl, B.A., Ma, L., Espinasa, L. and McGaugh, S.E. (2018)
- Porter, M.L. and Sumner‐Rooney, L. (2018)
- Pazza, R., Dergam, J.A. and Kavalco, K.F. (2018)
- Shiriagin, V. and Korsching, S.I. (2018)
- Espinasa, L., Hoese, G., Toulkeridis, T. and Toomey, R. (2018)
- Retaux, S. (2018)
- Riddle, M., Martineau, B., Peavey, M. and Tabin, C. (2018)
- Bilandzija, H., Abraham, L., Ma, L., Renner, K.J., & Jeffery, W.R. (2018)
- Blin, M., Tine, E., Meister, L., Elipot, Y., Bibliowicz, J., Espinasa, L. and Rétaux, S. (2018)
- Espinasa, L., Robinson, J. and Espinasa, M. (2018)
- Lloyd, E., Olive, C., Stahl, B.A., Jaggarda, J.B., Amaral, P., Dubouéa, E.R and Keene, A.C. (2018)
- Helena, B., Lindsey, A., Li, M., Kenneth, J.R. and William, R.J. (2018)
- Ahmad, H., Azizan, T.R.P.T., Noor, M.H.M., Hassim, H.A. and Daud, H.M. (2018)
- Herman, A., Brandvain, Y., Weagley, J., Jeffery, W.R., Keene, A.C., Kono, T.J., Bilandžija, H., Borowsky, R., Espinasa, L., O'Quin, K., Ornelas-García, C.P., Yoshizawa, M., Carlson, B, Maldonado E, Gross JB, Cartwright RA, Rohner N, Warren WC, McGaugh SE. (2018)
- Jaggard, J.B., Stahl, B.A., Lloyd, E., Prober, D.A., Duboue, E.R. and Keene, A.C. (2018)
- Kling, J. (2018)
- Keene, A.C.and Duboue, E.R. (2018)
- Kopp, J., Avasthi, S. and Espinasa, L. (2018)
- Torres-Paz,J., Hyacinthe, C., Pierre, C., Retaux, S. (2018)
- Chin, J.S.R., Gassant, C.E., Amaral, P.M., Lloyd, E., Stahl, B.A., Jaggard, J.B., Keene, A.C. and Duboue, E.R. (2018)
- Zhao, H., Di Mauro, G., Lungu-Mitea, S., Negrini, P., Guarino, A.M., Frigato, E., Braunbeck, T., Ma, H., Lamparter, T., Vallone, D., Bertolucci, C. and Foulkes, N.S. (2018)
- Kasumyana, A.O. and Marusova, E.A. (2018)
- Ojha, A. and Watve, M. (2018)
- Bussotti, S., Di Franco, A., Bianchi, C.N., Chevaldonné, P., Egea, L., Fanelli, E., Lejeusne, C., Musco, L., Navarro-Barranco, C., Pey, A. and Planes, S. (2018)
- Ornelas-García, P,. Pajares, S., Sosa-Jiménez, V.M., Rétaux, S. and Miranda-Gamboa, R.A. (2018)
- Ceinos, R., Frigato, E., Pagano, C., Fröhlich, N., Negrini, P., Cavallari, N., Vallone, D., Fuselli, S., Bertolucci, C. and Foulkes, N. (2018)
- Kasumyan, A.O. and Marusov, E.A. (2018)
- Stahl, B.A., Sears, C.R., Ma, L., Perkins, M., & Gross, J.B. (2018)
- Gore, A.V., Tomins K.A., Iben, J., Ma, L., Castranova, D., Davis, A.E., Parkhurst, A., Jeffery, W.R. and Weinstein, B.M. (2018)
- Gore, A., Jeffery, W., Retaux, S. and Rohner, N. (2018)
- Sovrano, V.A., Potrich, D., Foà, A. and Bertolucci, C. (2018)
- Stern, D.B and Crandall, K.A. (2018)
- Powers, A.K., Kaplan, S.A., Boggs, T.E. and Gross, J. (2018)
- Gore, A.V., Rohner, N., Rétaux, S. and Jeffery, W.R. (2018)
- Riddle, M.R., Aspiras, A., Damen, F., Hutchinson, J.N., Chinnapen, D. and Tabin, C.J. (2019)
- Borowsky, R., Luk, A. and Kim, R.S. (2019)
- Jeffery, W.R. (2019)
- Chin, J.S.R., Loomis, C.L., Phan, T.A.N., Albert, L.T., Chang, L.P., Keene, A.C. and Duboué, E.R. (2019)
- Potts, H.G., Stockdale, W.T., Choudhury, R.P. and Mommersteeg, M.T.M. (2019)
- Devos, L., Klee, F., Edouard, J., Simon, V., Legendre, L., Khallouki, N.E., Barbachou, S., Sohm, F. and Rétaux, S. (2019)
- Sears, C.R. (2019)
- Keene, A.C. (2019)
- Jaggard, J.B., Lloyd, E., Lopatto, A., Duboue, E.R. and Keene, A.C. (2019)
- Gil-Galvez, A., de la Calle Mustienes, E., Neto, A.., Bilandžija, H., Jeffery, W.R., Gómez-Skarmeta, J.L. (2019)
- Kling, J. (2019)
- Hu, Z., Lemieux, M. and Mommersteeg, M. (2019)
- Fernandes, C.S., Batalha, M.A., Bichuette, M.E. (2019)
- Reyes Corral, W.D. and Aguirre, W.E. (2019)
- Stockdale, W.T., Tang, J.L.Y., Hu, Z., Potts, H.G., Lemieux, M.E., Yamamoto, Y. and Mommersteeg, M.T.M. (2019)
- Borowsky, R. (2019)
- Powers, A.K., Boggs, T.E., Kaplan, S.A., Davis, E.M. and Gross, J.B. (2019)
- Leclercq, J., Torres-Paz, J. and Rétaux, S. (2019)
- Berning, D., Adams, H., Luc, H. and Gross, J.B. (2019)
- Policarpo, M., Fumey, J., Lafargeas, P., Legendre, L., Naquin, D., Thermes, C., Møller, P.R., Bernatchez, L., García-Machado, E., Rétaux, S. and Casane, D. (2019)
- Gallman, K., Rivera, D. and Soares, D. (2019)
- Warren, W.C., Koren, S., Meyer, A. and Rohner, N. (2019)
- Hyacinthe, C., Attia, J. and Retaux, S. (2019)
- Legendre, L., Pere, S. and Rétaux, S. (2019)
- Stahl, B.A., Peuß, R., McDole, B., Kenzior, A., Jaggard, J.B., Gaudenz, K., Krishnan, J., McGaugh, S.E., Duboue, E.R., Keene, A.C. and Rohner, N. (2019)
- Agnès, F., Devos, L. and Rétaux, S. (2019)
- McGaugh, S.E., Passow, C., Jaggard, J.B., Stahl, B. and Keene, A. (2019)
- Luc, H., Sears, C., Raczka, A. and Gross, J.B. (2019)
- Rétaux, S., Bibliowicz, Y., Blin, M., Elipot, Y., Espinasa, L., Hinaux, H. and Tine, E. (2019)
- Legendre, L., Guillet, B., Leguay, E., Meunier, E., Keck, N. and Sohm, F. (2019)
- Fernández, A.A., Evans, E.I. and Baumann, D.P. (2019)
- Pennisi, E. (2019)
- van der Laan, R. (2019)
- Culver, D.C. and Pipan, T. (2019)
- Wilkens, H (2019)
- Van der Weele, C.M. and Jeffery, W.R. (2019)
- Worsham, M., Fang, J. and Yoshizawa, M. (2019)
- Krishnan, J. and Rohner, N. (2019)
- O’Gorman, M., Lewis, P., Keene, A., Kowalko, J. and Duboué, E. (2019)
- Jazwinska, A. and Blanchoud, S. (2019)
- Pastana, M.N.L., Bockmann, F.A. and Datovo, A. (2019)
- Blin, M., Fumey, J., Père, S., Pierre, C., Torres-Paz, J., Simon, V., Espinasa, L. Legendre, L., Casane, D. and Rétaux, S. (2019)
- Fernandes, V.F.L., Hernandez, H., Doeden, N. and Yoshizawa, M. (2019)
- Devos, L., Edouard, J., Simon, V., Agnès, F., Sohm, F. and Rétaux, S. (2019)
- Ma, L. and Jeffery, W.R. (2019)
- Loomis, C., Peuß, R., Jaggard, J., Wang, Y., McKinney, S., Raftopoulos, S., Raftopoulos, A., Whu, D., Green, M., McGaugh, S.E., Rohner, N., Keene, A.C., Duboue, E.R. (2019)
- Loomis, C., Peuss, R., Jaggard, J.B., Wang, Y., McKinney, S.A., Raftopoulos, S.C., … Duboue, E.R. (2019)
- Lloyd, E., Olive, C., Stahl, B.A., Jaggard, J.B., Amaral, P., Duboué, E.R. and Keene, A.C. (2019)
- Boggs, T.E. and Gross, J.B. (2019)
- Atukorallaya, D., Bhatia, V. and Ratnayake, R. (2019)
- Jaggard, J., Lloyd, E., Loomis, C., Cook, C., Vega, P., McGaugh, S., Rohner, N., Bohrner, J., Duboue, E. and Keene, A.C. (2019)
- Hyacinthe, C., Attia, J. and Retaux, S. (2019)
- Barash, D.P. (2019)
- Duboue, E. (2019)
- Mogdans, J. (2019)
- McDole, B., Jaggard, J., Duboue, E. and Keene, A.C. (2019)
- Kibele, C.S., Montgomery, J.C. and Radford, C.A. (2019)
- Garduño-Sánchez, M.A.A. and Ornelas-García, C.P. (2019)
- Loomis, C., Peuss, R., Green, M., McGaugh, S.E., Rohner, N., Keene, A.C. and Duboue, E.R. (2019)
- Riddle, M., Damen, F., Aspiras, A., Tabin, J., McGaugh, S. and Tabin, C. (2019)
- Simon, V., Hyacinthe, C. and Retaux, S. (2019)
- Peuß, R., Box, A.C., Wang, Y., Chen, S., Krishnan, J., Tsuchiya, D., Peak, A., Perera, A., Slaughter, B. and Rohner, N. (2019)
- Keene, A.C. and Appelbaum, L. (2019)
- Maximino, C., do Carmo Silva, R.X., dos Santos Campos, K., de Oliveira, J.S., Rocha, S.P., Pyterson, M.P., dos Santos Souza, D.P., Feitosa, L.M., Ikeda, S.R., Pimentel, A.F.N., Pâmila N. F. (2019)
- Paz, A., McDole, B., Duboue, E. and Keene, A.C. (2019)
- Riddle, M.R. and Tabin, C.J. (2019)
- Torres-Paz, J., Leclercq. J. and Rétaux, S. (2019)
- McNeill-Jewer, C.A., Reinhardt, E.G., Collins, S., Kovacs, S., Chan, W.M., Devos and LeMaillot, F.C. (2019)
- Kowalko, J.E. (2019)
- van der Weele, C.M. and Jeffery, W.R. (2019)
- McGaugh, S.E., Passow, C.N., Jaggard, J.B., Stahl, B.A. and Keene, A.C. (2019)
- Mcgaugh, S.E., Weaver, S., Gilbertson, E.N., Garrett, B., Rudeen, M.I., Grieb, S., Roberts, J., Donny, A., Marchetto, P. and Gluesenkamp, A,G. (2019)
- Emam, A., Yoffe, M., Cardona, H. and Soares, D. (2019)
- Krishnan, J. and Rohner, N. (2019)
- Bilandžija, H., Hollifield, B., Steck, M., Meng, G., Ng, M., Bedek, J., Ćetković, H., Porter, M., Renner, K.J. ands Jeffery, W.R. (2019)
- Bilandžija, H., Hollifield, B., Steck, M., Meng, G., Ng, M., Koch, A.D., Gračan, R., Ćetković, H., Porter, M.L., Renner, K.J. and Jeffery, W.R. (2019)
- Yoshizawa, M., Valdez, C., Balaan, C., Lactaoen, K., Kato, J., Ito, M., Yew, J. and Iwashita, M. (2019)
- Sifuentes-Romero I. and Kowalko, J.E. (2019)
- Coppola, J. and Espinasa, L. (2019)
- Hernández, J., Garita-Alvarado, C.A. and Ornelas-García, C.P. (2019)
- Stahl, B.A., Peuß, R., McDole, B., Kenzior, A., Jaggard, J.B., Gaudenz, K., Krishnan, J., McGaugh, S.E., Duboue, E.R., Keene, A.C and Rohner, N. (2019)
- Phelps, A. and Gross, J. (2019)
- Imarazene, B., Herpin, A., Feron, R., Postlethwait, J.H., Schartl, M., Retaux, S. and Guiguen, Y. (2019)
- Espinasa, L., Retaux, S., Yoshisawa, M., Heintz, C. and Balogh-Robinson, R. (2019)
- Jeffery, W.R., Ma, L., Shi, J., Ng, M. and Gore,A. (2019)
- Mogdans, J. (2019)
- McGaugh, S.E., Weaver, S., Gilbertson, E.N., Garrett, B., Rudeen, M.L., Grieb, S., Roberts, J., Donny, A., Marchetto, P. and Gluesenkamp, A.G. (2019)
- Piscor, D., Pozzobon, A.P.B., Fernandes, C.A., Centofante, L, and Parise-Maltempi, P.P. (2019)
- Yoffe, M., Patel, K., Palia, E., Kolawole, S., Streets, A., Haspel, G. and Soares, D. (2019)
- Palluzzi, T., Rowland, R. and Gross, J. (2019)
- Ornelas-García, C.P., Pajares, S., Sosa-Jiménez, V.M., Rétaux, S. and Gamboa-Miranda, R. (2019)
- Torres-Paz, J., Leclercq, J. and Rétaux, S. (2019)
- Stahl, B.A., Peuß, R., McDole, B., Kenzior, A., Jaggard, J.B., Gaudenz, K., Krishnan, J., McGaugh, S.E., Duboue, E.R., Keene, A.C. and Rohner, N. (2019)
- Powers, A.K., Garita-Alvarado, C., Rodiles, R., Berning, D.J., Gross, J.B. and Ornelas-García, C.P. (2019)
- Stahl, B.A., Jaggard, J.B., Chin, J.S., Kowalko, J.E., Keene, A.C. and Duboué, E.R. (2019)
- Worsham, M., Fernandes, V.F.L., Settle, A., Balaan, C., Lactaoen, K., Tuttle, L.J., Iwashita, M. and Yoshizawa, M. (2019)
- Gluesenkamp, A. and McGaugh, S. (2019)
- Atukorala, A.D.S., Bhatia, V. and Ratnayake, R. (2019)
- Kindl, G.H. and O’Quin, K.E. (2019)
- Peuß, R., Zakibe, Z., Krishnan, J., Merryman, M.S., Baumann, D.P. and Rohner, N. (2019)
- Betancur-R.R., Arcila, D., Vari, R.P. et al. (2019)
- Pierre, C., Torres-Paz, J., Froc, C., Callebert, J. and Rétaux, S. (2019)
- Stockdale, W.T., Deal, O., Heintz, C., Rohner, N., and Mommersteeg, M.T.M. (2019)
- Shimobayashi, M., Shetty, S., Frei, I.C., Wölnerhanssen, B.K., Weissenberger, D., Dietz, N., Thomas, A., Ritz, D., Meyer-Gerspach, A.C., Maier, T., Hay, N., Peterli, R., Rohner, N. (2019)
- Xiong, S., Krishnan, J., Peuss, R. and Rohner, N. (2019)
- Kasumyan, A.O. and Vinogradskaya, M.I. (2019)
- Simon, N., Fujita, S., Porter, M. and Yoshizawa, M. (2019)
- Gore, A., Swearer, A., Iben, J. and Weinstein, B. (2019)
- Blin, M. and Rétaux, S. (2019)
- Cao, J. and Cheng, X. (2020)
- Ingalls, A., Merryman, S., Baumann, D.P. (2020)
- Marandel, L., Plagnes-Juan, E., Marchand, M., Callet, T., Dias, K., Terrier, F., Père, S., Vernier, L., Panserat, S. and Rétaux, S. (2020)
- Policarpo, M., Fumey, J., Lafargeas, P., Naquin, D., Thermes, C., Naville, M., Dechaud, C., Volff, J.-M., Cabau, C., Klopp, C., Rask Møller, P., Bernatchez, L., García-Machado, E., Rétaux, S. and Casane, D. (2020)
- Ma, L., Gore, A.V., Castranova, D., Shi, J., Ng, M., Tomins, K.A., van der Weele, C.M., Weinstein, B.M. and Jeffery, W.R. (2020)
- Warren, W.C., Boggs, T.E., Borowsky, R., Carlson, B.M., Ferrufino, E., Gross, J.B., Hillier, L., Hu, Z., Keene, A.C., Kenzior, A., et al. (2020)
- Peuss, R., Box, A.C., Chen S., Wang Y., Tsuchiya D., Persons J. L., . . . and Rohner N. (2020)
- Yoffe, M., Patel, K., Palia, E., Kolawole, S., Streets, A. Haspel, G. and Soares, D. (2020)
- Powers, A.K., Berning, D.J. and Gross, J.B. (2020)
- Espinasa, L., Ornelas-García, C.P., Legendre, L., Rétaux, S., Best, A., Gamboa-Miranda, R., Espinosa-Pérez, H. and Sprouse, P. (2020)
- Ingalls, A., Merryman, S., Baumann, D.P. (2020)
- Blin, M., Fumey, J., Lejeune, C., Policarpo, M., JLeclercq, J., Père, S., Torres-Paz, J., Pierre, C., Imarazene, B. and Rétaux, S. (2020)
- Cutlip, C. (2020)
- Espinasa, L., Ornelas-García,C. P., Legendre, L., Rétaux, S., Best, A., Gamboa-Miranda, R., Espinosa-Pérez, H., and Sprouse, P. (2020)
- Medley, J.K., Persons, J., Peuß, R., Olsen, L., Xiong, S. and Rohner, N. (2020)
- Pierre, C., Pradère, N., Froc, C., Ornelas-García, P., Callebert, J. and Rétaux. S. (2020)
- Minchin, J.E.N. (2020)
- McGaugh, S.E., Kowalko, J., Duboue, E.R., Lewis, P., Franz-Odendaal, T.A., Rohner, N., Gross, J.B. and Keene, A.C. (2020)
- Wilkens, H. (2020)
- Rohner, N. (2020)
- Terán, G.E., Benitez, M.F. and Mirande, J.M. (2020)
- Patch, A., Paz, A., Holt, K., Duboue, E., Kowalko, J., Keene, A. and Fily, Y. (2020)
- Phelps, A.N., Luc, H.M. and Gross, J.B. (2020)
- Jeffery, W.R. (2020)
- Kowalko, J.E., Franz‐Odendaal, T.A. and Rohner, N. (2020)
- Kowalko, J.E., Franz-Odendaal, T.A. and Rohner, N. (2020)
- Mercado-Silva, N., Ornelas-Garcia, C.P., Schmitter-Soto, J.J., Gidmark, N.J. and Simons, A.M. (2020)
- Riddle, M.R., Aspiras, A.C., Damen, F., Hutchinson, J.N., Chinnapen, D.J.F., Tabin, J. and Tabin, C.J. (2020)
- Ornelas, P., Gonzalez, E.G., Tautz, D. and Doadrio, I. (2020)
- Warren, M.L. and Burr, B.M. (2020)
- Torres-Paz, J. and Rétaux, S. (2020)
- Chin, J.S.R., Loomis, C.L., Albert, L.T., Medina‐Trenche, S., Kowalko, J., Keene, A.C. and Duboué, E.R. (2020)
- Gallman, K., Fortune, E., Rivera, D. and Soares, D. (2020)
- Franz-Odendaal, T.A. (2020)
- Krishnan, J., Persons, J.L., Peuß, R., Hassan, H., Kenzior, A., Xiong, S., Olsen, L., Maldonado, E., Kowalko, J.E. and Rohner, N. (2020)
- Ma, L., Ng, M., van der Weele, C.M., Yoshizawa, M. and Jeffery, W.R. (2020)
- Kowalko, J.E. (2020)
- Jandzik, D. and Stock, D.W. (2020)
- Riddle, M.R., Aspiras, A.C., Gaudenz, K., Peuß, R., Sung, J.Y., Martineau, B., Peavey, M., Box, A.C., Tabin, J.A., McGaugh, S., Borowsky, R., Tabin, C.J. and Rohner, N. (2020)
- Gross, J.B. and Powers, A.K. (2020)
- Sifuentes‐Romero, I., Ferrufino, E., Thakur, S., Laboissonniere, L.A., Solomon, M., Smith, C.L., Keene, A.C., Trimarchi, J.M. and Kowalko, J.E. (2020)
- Espinasa, L., Ornelas-García, C.P., Legendre, L., Rétaux, S., Best, A., Gamboa-Miranda, R., Espinosa-Pérez, H. and Sprouse, P. (2020)
- Paz, A., McDole, B., Kowalko, J.E., Duboue, E.R. and Keene, A.C. (2020)
- McGaugh, S.E., Passow, C.N., Jaggard, J.B., Stahl, B.A. and Keene, A.C. (2020)
- Sears, C.R., Boggs, T.E. and Gross, J.B. (2020)
- Ma, L., Ng, M., Shi, J., Gore, A.V., Castranova, D., Weinstein, B.M. and Jeffery, W.R. (2020)
- Emam, A., Yoffe, M., Cardona, H. and Soares, D. (2020)
- Policarpo, M., Fumey, J., Lafargeas, P., Naquin, D., Thermes, C., Naville, M., Dechaud, C., Volff, J.-M., Cabau, C., Klopp, C., Rask Møller, P., Bernatchez, L., García-Machado, E., Rétaux, S. and Casane, D. (2020)
- Bilandžija H., Hollifield B., Steck M., Meng G., Ng M., Koch A. D, Gračan, R., Ćetković, H., Porter, M.L., Renner, K.J. and Jeffery, W. (2020)
- Pierre, C. (2020)
- Jaggard, J.B., Lloyd, E., Yuiska, A., Patch, A., Fily, Y., Kowalko, J.E., Appelbaum, L., Duboue, E.R. and Keene, A.C. (2020)
- Gimhani, N. and Atukorallaya, D. (2020)
- Krishnan, J., Seidel, C.W., Zhang, N., Van Campen, J., Peuß, R., Xiong, S., Kenzior, A., Li, H., Conaway, J.W. and Rohner, N. (2020)
- Maldonado, E., Rangel‐Huerta, E., Rodriguez‐Salazar, E., Pereida‐Jaramillo, E. and Martínez‐Torres, A. (2020)
- Chin, J.S.R., Loomis, C.L., Albert, L.T., Medina-Trenche, S., Kowalko, J., Keene, A.C. and Duboué, E.R. (2020)
- Leclercq, J., Pierre, C., Simon, V. and Blin, M. (2020)
- Mack, K.L., Jaggard, J.B., Persons, J.L., Passow, C.N., Stanhope, B.A., Ferrufino, E., Tsuchiya, D., Smith, S.E., Slaughter, B.D., Kowalko, J., Rohner, N., Keene, A.C. and McGaugh, S.E. (2020)
- Kowalko, J. (2020)
- Riddle, M.R., Damen, F., Aspiras, A., Tabin, J.A., McGaugh, S. and Tabin, C.J. (2020)
- Powers, A.K., Boggs, T.E.and Gross, J.B. (2020)
- Peuß, R., Box, A.C., Chen, S., Wang, Y., Tsuchiya, D., Persons, J.L., Kenzior, A., Maldonado, E., Krishnan, J., Scharsack, J.P., Slaughter, B.D. and Rohner, N. (2020)
- Pierre, C., Pradère, N., Froc, C., Ornelas-García, P., Callebert, J. and Rétaux, S. (2020)
- Moran, R.L., Jaggard, J.B., Roback, E.Y., Rohner, N., Kowalko, J.E., Ornelas-García, C.P., McGaugh, S.E. and Keene, A.C. (2021)
- Warren, W.C., Boggs, T.E., Borowsky, R., Carlson, B.M., Ferrufino, E., Gross, J.B., Hillier, L., Hu, Z., Keene, A.C., Kenzior, A. and Kenzior, A. and Kowalko, J.E. (2021)
- Oliva, C., Hinz, N.K., Robinson, W., Barrett Thompson, A.M., Booth, J., Crisostomo, L.M., Zanineli, S., Tanner, M., Lloyd, E., O’Gorman, M., McDole, B., Paz, A., Kozol, R. et al. (2021)
- Mack, K.L., Jaggard, J.B., Persons, J.L., Roback, E.Y., Passow, C.N., Stanhope, B.A., Ferrufino, E., Tsuchiya, D. Smith, S.E., Slaughter, B.D. et al. (2021)
- Devos, L., Agnès, F., Edouard, J., Simon, V., Legendre, L., El Khallouki, N., Barbachou, S., Sohm, F. and Rétaux, S. (2021)
- Gimhani, N. and Atukorallaya, D. (2021)
- Xiong, S., Wang, W., Kenzior, A., Olsen, L., Krishnan, J., Persons, J., Medley, K., Peuß, R., Wang, Y., Chen, S., Zhang, N., Thomas, N., Miles, J.M., Sánchez Alvarado, A. and Rohner, N. (2021)
- Torres-Paz, J. and Rétaux, S. (2021)
- Boggs, T. and Gross, J. (2021)
- Imarazene, B., Du, K., Beille, S., Jouanno, E., Feron, R., Pan, Q., Torres-Paz J., Lopez-Roques, C., Castinel,A., Gil, L., Kuchly, C., Donnadieu, C., Parrinello,H., Journot, L., Cabau, C., Zahm, M., Klopp, C., Pavlica, T., Al-Rikabi, A., Liehr, T., Sima (2021)
- Agnès, F., Torres-Paz, J., Michel, P. and Rétaux, S. (2021)
- Ma, L., Ng, M., Shi, J., Gore, A.V., Castranova, D., Weinstein, B.M. and Jeffery, W.R. (2021)
- Tanvir, Z., Rivera, D., Severi, K.E., Haspel, G. and Soares, D. (2021)
- Iwashita, M. and Yoshizawa, M. (2021)
- Ma, L., Dessiatoun, R., Shi, J. and Jeffery, W.R. (2021)
- Riddle, M.R., Aspiras, A., Damen, F., McGaugh, S., Tabin, J.A. and Tabin, C.J. (2021)
- Eschmeyer, W.N. and Van der Laan, R. and Fricke, R. (2021)
- Borowsky, R. (2021)
- O'Gorman, M., Thakur, S., Imrie, G., Moran, R.L., Choy, S., Sifuentes-Romero, I., Bilandžija, H., Renner, K.J., Duboué, E., Rohner, N., McGaugh, S.E., Keene, A.C. and Kowalko, J.E. (2021)
- Zhao, H., Li, H., Du, J., Di Mauro, G., Lungu-Mitea, S., Geyer, N., Vallone, D., Bertolucci, C. and Foulkes, N.S. (2021)
- Wilkens, H. (2021)
- Garita-Alvarado, C.A., Garduño-Sánchez, M., Barluenga, M. and Ornelas-García, C.P. (2021)
- Kistner, A.R., Enriquez, M.S., Michels, N.O. and Mensinger, A.F. (2021)
- Policarpo, M., Laurenti, P., García-Machado, E., Metcalfe, C., Rétaux, S. and Casane, D. (2021)
- Espinasa, L., Heintz, C., Rétaux, S., Yoshisawa, M., Agnès, F., Ornelas-Garcia, P. and Balogh-Robinson, R. (2021)
- Iwashita, M. and Yoshizawa, M. (2021)
- Garita-Alvarado, C.A., Garduño-Sánchez, M., Barluenga, M. and Ornelas-García, C.P. (2021)
- Jandzik, D. and Stock, D.W. (2021)
- Wilson, E.J., Tobler, M., Riesch, R., Martínez‑García, L. and García‑De León, F.J. (2021)
- Devos, L., Agnès, F., Edouard, J., Simon, V., Legendre, L., El Khallouki, N., Barbachou S., Sohm, F. and Rétaux, S. (2021)
- Potts, H.G., Stockdale,W.T. and Mommersteeg, M.T.M. (2021)
- Olsen, L., Thum, E. and Rohner, N. (2021)
- Broadfoot, M.V. (2021)
- Torres-Paz, J. and Retaux, S. (2021)
- Krishnan, J., Seidel, C.W., Zhang, N., VanCampen, J., Peuss, R., Xiong, S., Kenzior, A., Li, H., Conaway, J.W. and Rohner, N. (2021)
- Imarazene, B., Beille, S., Jouanno, E., Branthonne, A., Thermes, V., Thomas, M., Herpin, A., Rétaux, S. and Guiguen, Y. (2021)
- Pérez-Rodríguez, R., Esquivel-Bobadilla, S., Orozco-Ruíz, A.M., Olivas-Hernández, Jé.L. and García-De León, F.J. (2021)
- Leclercq, J. (2022)
- Medley, J.K., Persons, J., Biswas, T., Olsen, L., Peuß, R., Krishnan, J., Xiong, S. and Rohner, N. (2022)
- Leclercq, J., Torres-Paz, J. and Rétaux, S. (2022)
- Agnès, F., Torres-Paz, J., Michel, O. and Rétaux, S. (2022)
- Lloyd, E., McDole, B., Privat, M., Jaggard, J.B., Duboue, E.R., Sumbre, G. and Keene, A.C. (2022)
- Lloyd, E., McDole, B., Privat, M., Jaggard, J.B., Duboué, E., Sumbre, G. and Keene, A. (2022)
- Nwosu, U. (2022)
- Boggs, T. and Gross, J.B. (2022)
- Agnès, F., Torres-Paz, J., Michel, P. and Rétaux, S. (2022)
- Garduno-Sanchez, M., Hernandez-Lozano, J., Moran, R., Miranda-Gamboa, R., Gross, J., Rohner, N., Elliott, W.R., Miller, J., Lozano-Vilano, L., McGaugh, S. and Ornelas-Garcia, C. (2022)
- Krishnan, J., Wang, Y., Kenzior, O., Hassan, H., Olsen, L., Tsuchiya, D., Kenzior, A., Peuß, R., Xiong, S., Wang, Y., Zhao, C. and Rohner, N. (2022)
- Rodriguez-Morales, R., Gonzalez-Lerma, P., Yuiska, A., Heon Han, J., Guerra, Y., Crisostomo, L., Keene, A.C., Duboue, E.R. and Kowalko, J.E. (2022)
- Bhatia, V., de Jesus, V.C., Shaik, F.A., Jaggupilli, A., Singh, N., Chelikani, P. and Atukorallaya, D. (2022)
- Powers, A., Berning, D., Boggs, T., Hamm, A. and Gross, J. (2022)
- Pennington, A. (2022)
- van der Weele, C. and Jeffery, W.R. (2022)
- Rodríguez-Ballesteros, V., Mendoza-Garfias, B., Ulloa-Arvizu, R., Balcazar, A. and Ornelas-García, C.P. (2022)
- Rodriguez-Morales, R., Gonzalez-Lerma, P., Yuiska, A., Heon Han, J., Guerra, Y., Crisostomo, L., Keene, A.C., Duboue, E.R. and Kowalko, J. (2022)
- Powers, A.K and Tabin, C. (2022)
- Oliva, C., Hinz, N.K., Robinson, W., Barrett Thompson, A.M., Booth, J., Crisostomo, L.M., Zanineli, S., Tanner, M., Lloyd, E., O’Gorman, M., McDole, B., Paz, A., Kozol, R. et al. (2022)
- Olsen, L., Gluesenkamp, A., Mager, E., Coughlin, D. and Rohner, N. (2022)
- Fernandes, V.F.L., Glaser, Y., Iwashita, M. and Yoshizawa, M. (2022)
- Berning, D. and Gross, J.B. (2022)
- Agnes, F., Torres-Paz, J., Michel, P. and Rétaux, S. (2022)
- Duboue, E.R., Kowalko, J.E. and Keene, A.C. (2022)
- Callier, V. (2022)
- Yoshizawa, M., Balmilero-Unciano, R., Valdez, C., Lactaoen, K., Kato, J., Ito, M., Clarke, K. and Iwashita, M. (2022)
- Legendre, L., Rode, J., Germon, I., Pavie, M., Quiviger, C., Policarpo, M., Leclercq, J., Père, S., Fumey, J., Hyacinthe, C., Ornelas-García, P., Espinasa, L., Rétaux, S. and Casane, D. (2022)
- Goes, C.A.G., dos Santos, R.Z., Aguiar, W.R.C., Alves, D.C.V., Silva, D.M.Z.dA., Foresti, F., Oliveira, C., Utsunomia, R. and Porto-Foresti, F. (2022)
- Jeffery, W.R. Ma, L., Ng, M., Shi, J. and Gore, A.V. (2022)
- Kuhn, J., Azari, S. and Volkoff, H. (2022)
- Potts, H.G., Lemieux, M.E., Rice, E.S., Warren,W., Choudhury, R.P. and Mommersteeg, M.T.M. (2022)
- Xiong, S., Wang, W., Kenzior, A., Olsen, L., Krishnan, J., Persons, J., Medley, K., Peuß, R., Wang, Y., Chen, S., Zhang, N., Thomas, N., Miles, J. M., Alvarado, A. S. and Rohner, N. (2022)
- Espinasa, L. and Diamant, R. (2022)
- Paz, A., Holt, K., Duboué, E., Fily, Y. and Kowalko, J. (2022)
- Espinasa, L., Collins, E., Ornelas García, C.P., Rétaux, S., Rohner, N. and Rutkowski, J. (2022)
- Choy, S., Polyakov, E., Thakur, S., Lloyd, E., Keene, A. and Kowalko, J. (2022)
- Vogt, G. (2022)
- Maderspacher, F. (2022)
- Moran, R.L., Jaggard, J.B., Roback, E.Y., Kenzior, A., Rohner, N., Kowalko, J.E., Ornelas-García, C.P., McGaugh, S.E. and Keene, A.C. (2022)
- Berning, D. (2022)
- Lunghi, E. and Bilandžija, H. (2022)
- Rodríguez-Morales, R., Gonzalez, P., Guerra, Y., Crisostomo, L., Pileggi, B., Kimmel, A. and Kowalko, J. (2022)
- Tomkins, J.P., Arledge, S. and Guliuzza, R.J. (2022)
- Hutton, P., Rodríguez-Morales, R., Ferrufino, E., Lloyd, E., Dubuoé, E.R., Kowalko, J.E. and Keene, A.C. (2022)
- Lesku, J.A. and Rattenborg, N.C. (2022)
- Perry, A., McGaugh, S.E., Keene, A.C. and Blackmon, H. (2022)
- Wilson, E.J., Tobler, M., Riesch, R., Martínez-García, L. and García-De León, F.J. (2022)
- Santacruz, A., Hernández-Mena, D., Miranda-Gamboa, R.A., Pérez-Ponce de León, G. and Ornelas-Garcíai, P. (2022)
- Leal, F. and Tabin, C.J. (2022)
- Hyacinthe, C. and Tabin, C. (2022)
- Roberts, R.J.B., Pop, S. and Prieto-Godino, L.L. (2022)
- Hyacinthe, C., Attia, J., Schutz, E., Casane, D. and Rétaux, S. (2022)
- Shafer, M.E.R., Sawh, A.N. and Schier, A.F. (2022)
- Tanvir, Z., Rivera, D., Severi, K.E., Haspel, G. and Soares, D. (2022)
- Espinasa, L., Diamant, R., Mesquita, M., Lindquist, J.M., Powers, A.M. and Helmreich, J. (2022)
- Lloyd, E., Chouuk, B., Conith, A., Albertson, R.C. and Keene, A.C. (2022)
- Espinasa, L., Rohner, N. and Rétaux, S. (2022)
- Schartl, M. (2022)
- Soares, D. (2022)
- Kozol, R.V., Conith, A.J., Yuiska, A., Cree-Newman, A., Tolentino, B., Banesh, K., Paz, A., Lloyd, E., Kowalko, J.E., Keene, A.C., R. Albertson, C. and Duboue, E.R. (2022)
- Fily, Y., Patch, A., Paz, A., Holt, K.J., Duboué, E.R., Keene, A.C. and Kowalko, J.E. (2022)
- Boggs, T.E., Friedman, J.S. and Gross, J.B. (2022)
- Mogdans, J. (2022)
- Tobler, M. (2022)
- Rodriguez-Santiago, M., Jordan, A. and Hofmann, H.A. (2022)
- Anonymous (2022)
- Gross, J.B., and Powers, A.K. (2022)
- Olsen, L., Levy, M., Medley, J.K., Hassan, H., Alexander, R., Wilcock, E., Yi, K., Florens, L., McKinney, S., Peuß, R., Persons, J., Kenzior, A., Maldonado, E., Gluesenkamp, A. et al. (2022)
- Lunsford, E.T., Paz, A., Keene, A.C. and Liao, J.C. (2022)
- Leclercq, J., Torres-Paz, J., Policarpo, M., Agnès, F. and Rétaux, S. (2022)
- Krishnan, J., Wang, Y., Kenzior, O., Huzaifa, H., Olsen, L., Tsuchiya, D., Wang, Y., Zhao, C. and Rohner, N. (2022)
- Reyes Pérez, R.I. (2022)
- Sumbre, G. (2022)
- Patch, A., Paz, A., Holt, K.J., Duboué, E.R., Keene, A.C., Kowalko, J.E. and Fily, Y. (2022)
- Anonymous (2022)
- Rice, E. Warren, W.C., Carroll, R., Keene, A. and Rohner, N. (2022)
- Lunsford, E.T. (2022)
- Porfiri, M., Zhang, P. and Peterson, S.D. (2022)
- Jeffery, W. (2022)
- Baumann, D.P. and Ingalls, A. (2022)
- Stanton, D., Justin, H.S. and Reitzel, A.M. (2022)
- Thakur, S., Tuyet Tra, N. and Cummings, M. (2022)
- Perry, A. McGaugh, S.E., Keene, A.C. and Blackmon, H. (2022)
- Páscoa, I., Fonseca, E., Ferraz, R., Machado, A.M., Conrado, F., Ruivo, R., Cunha, I. and Castro, L.F.C. (2022)
- van der Weele C.M. and Jeffery, W.R. (2022)
- Silva. P., Zavareh, P.A. and Atukorala, D. (2022)
- Hendrickson, D. (2022)
- Baratti, G., Potrich, D., Lee, S.A., Morandi-Raikova, A. and Sovrano, V.A. (2022)
- Biswas, T., Paulson, A., Hall, K, and Rohner, N. (2022)
- Kemeny, R. (2022)
- Enriquez, M.S. (2022)
- Perry, A., McGaugh, S.E., Keene, A.C. and Blackmon, H. (2022)
- Lunsford, E.T., Paz, A., Keene, A.C. and Liao, J.C. (2022)
- Fernandes, V.F.L., Espinasa, L., Glaser, Y., Iwashita, M., Sadowski, P. and Yoshizawa, M. (2022)
- Bronner, M. (2022)
- Smith, D.G. and Bowman, I.A. (2022)
- Riddle, M., Guerra, D.P., Aspiras, A., Chinnapen, D., Damen, F., Hutchinson, J.N., McGaugh, S., Tabin, J. and Tabin, C. (2022)
- Moran, R.L., Jaggard, J.B., Roback, E.Y., Kenzior, A., Rohner, N., Kowalko, J.E., Ornelas-García, C.P., McGaugh, S.E. and Keene, A.C. (2022)
- Lesser, E. (2022)
- Martik, M. (2022)
- Powers, A.K., Hyacinthe, C., Riddle, M., Kim, Y.K., Amaismeier, A., Thiel, K., Martineau, B., Ferrante, E., Moran, R., McGaugh, S., Boggs, T., Gross, J.B. and Tabin, C.J. (2022)
- Pierre, C., Callebert, J., Launay, J.M. and Rétaux, S. (2022)
- Krishnan, J., Seidel, C.W., Zhang, N., Singh, N.P., VanCampen, J., Peuß, R., Xiong, S., Kenzior, A., Li, H., Conaway. J.W. and Rohner, N. (2022)
- Krishnan, J., Seidel, C.W., Zhang, N., Singh, N.P., Van Campen, J., Peuß, R., Xiong, S., Kenzior, A., Li, H., Conaway, J.W. and Rohner, N. (2022)
- Iwashita, M., Tran, A., Wong, M., Lee, R. and Yoshizawa, M. (2022)
- Rétaux, S. (2022)
- Kozol, R.A., Conith, A.J., Yuiska, A., Cree-Newman, A., Tolentino, B., Banesh, K., Paz, A., Lloyd, E., Kowalko, J.E., Keene, A.C., Albertson, R.C. and Duboue, E.R. (2022)
- Retaux, S., Attia, J., Blin, M., Casane, D., Espinasa, L., Hyacinthe, C., Imarazene, B., Leclercq, J., Legendre, L., Père, S., Pierre, C., Policarpo, M., Torres-Paz, J., and Simon, V. (2022)
- Borowsky, R. and Chen, H. (2022)
- Espinasa, L. and Pech, A. (2023)
- Di Lorenzo, T., Avramov, M., Galassi, D.M.P., Iepure, S., Mammola, S., Reboleira, A.S.P.S and Hervant, F. (2023)
- Santacruz, A., Hernández-Mena, D., Miranda-Gamboa, R., Pérez-Ponce De León, G. and Ornelas-García, P. (2023)
- Pavlova, V.V. and Krylov, V.V. (2023)
- Garduño-Sánchez, M., Hernández-Lozano, J., Moran, R.L., Miranda-Gamboa, R., Gross, J.B., Rohner, N., Elliott, W.R., Miller, J., Lozano-Vilano, L., McGaugh, S.E. and Ornelas-García, C.P. (2023)
- Casane, D., Saclier, N., Policarpo, M., François, C. and Lefébure, T. (2023)
- Lloyd, E., Privat, M., Sumbre, G., Duboue, E.R. and Keene, A.C. (2023)
- Policarpo, M., Legendre, L., Germon, I., Lafargeas, P., Espinasa, L., Rétaux, S. and Casane, D. (2023)
- Zhang, J.H., Long, R., Jing, Y.Y., Zhang, P., Xu, Y., Xiong, W., Zhu, Y.Q. and Luo, Y.P. (2023)
- Garduño-Sánchez, M.A.A., de Jesus-Bonilla, V., Perea, S., Miranda-Gamboa, R., Herrera- García, A., de la Maza Benignos, M. and Ornelas-García, C.P. (2023)
- Bucher, L. and Gross, J. (2023)
- Jiménez, A.G., Nash-Braun, E. and Meyers, J.R. (2023)
- Borowsky, R.L. (2023)
- Lomheim, H.J., Rodas, L.R., Mulla, L., Freeborn, L., Sun, D.A., Sanders, S.A. and Protas, M.E. (2023)
- Culver, D.C., Kowalko, J.E. and Pipan, T. (2023)
- Kozol, R.A., Yuiska, A., Han, J.H., Tolentino, B., Lopatto, A., Lewis, P., Paz, A., Keene, A.C., Kowalko, J.E. and Duboue, E.R. (2023)
- Batista da Silva, I., Barbosa, D.A., Kavalco, K.F., Nunes, L.R., Pasa, R. and Menegidio, F.B. (2023)
- Jiménez, A.G., Nash-Braun, E. and Meyers, J.R. (2023)
- Gil Galvez, A. (2023)
- Baumann, D.P. and Ingalls, A. (2023)
- Yang, X., Song, Y., Zhang, R., Yu, M., Guo, X., Guo, H., Du, X., Sun, S., Li, C., Mao, X., Fan, G. and Liu, X. (2023)
- Lakhiani, R., Shanavas, S. and Melnattur, K. (2023)
- Berning, D. and Gross, J.B. (2023)
- Olsen, L., Krishnan, J., Banks, C., Hassan, H. and Rohner, N. (2023)
- Blin, M., Rétaux, S. and Torres-Paz, J. (2023)
- Legendre, L., Rode, J., Germon, I., Pavie, M., Quiviger, C., Policarpo, M., Leclercq, J., Père, S., Fumey, J. Hyacinthe, C. et al. (2023)
- Castillo-Casas, J.M., Caño-Carrillo, S., Sánchez-Fernández, C., Franco, D. and Lozano-Velasco, E. (2023)
- Gross, J.B. and Powers, A.K. (2023)
- Kuball, K., Fernandes, V.F.L, Takagi, D. and Yoshizawa, M. (2023)
- Cardozo, M.J., Sánchez-Bustamante, E. and Bovolenta, P. (2023)
- Sifuentes-Romero, I., Ferrufino, E. and Kowalko, J.E. (2023)
- Blin, M., Valay, L., Kuratko, M., Pavie, M. and Rétaux, S. (2023)
- Hamm, A.R. (2023)
- Hyacinthe, C., Attia, J., Schutz, E., Lego, L., Casane, D. and Retaux, S. (2023)
- Arcila, D., Rincon-Sandoval, M., Hanson, W., Hart, P.B., González, V.L., Betancur-R, R. and Bichuette, M.E. (2023)
- Olsen, L. plus 17 others (2023)
- Lloyd, E., Kozol, R.A., Duboue, E.R. and Keene, A.C. (2023)
- Iwashita, M., Tran, A., Garcia, M., Cashon, J., Burbano, D., Salgado, V., Hasegawa, M., Balmilero‑Unciano, R., Politan, K., Wong, M., Lee, R.W.Y. and Yoshizawa, M. (2023)
- Weinberger, M. and Riley, P.R. (2023)
- Moran, R.L., Richards, E.J., Ornelas-García, C.P., Gross, J.B., Donny, A., Wiese, J., Keene, A.C., Kowalko, J.E., Rohner, N. and McGaugh, S.E. (2023)
- Biswas, T., Rajendran, N., Hassan, H., Zhao, C. and Rohner, N. (2023)
- Gross, J.B., Berning, D., Phelps, A. and Luc, H. (2023)
- Hutton, P., Lloyd, E., Dotson, M. and Keene, A.C. (2023)
- Alunni, A., Pierre, C., Torres-Paz, J., Clairet, N., Langlumé, A., Pavie, M., Escoffier-Pirouelle, T., Leblanc, M., Blin, M. and Rétaux, S. (2023)
- Wilkens, H. (2023)
- Staeger, S. (2023)
- Espinasa, L., Pavie, M. and Rétaux, S. (2023)
- Atukorallaya, D., Bhatia, V. and Gonzales, J. (2023)
- Iwashita, M. and Yoshizawa, M. (2023)
- Boyle, M.J., Arledge, S., Thomas, B., Tomkins, J.P. and Guliuzza, R.J. (2023)
- Strand, S.H. (2023)
- Legendre, L., Espinasa, L., Lacaille-Múzquiz, J.-L., Alaniz-Garfia, G., Ornelas-García, P. and Rétaux, S. (2023)
- Olsen, L., Krishnan, J., Banks, C., Hassan, H. and Rohner, N. (2023)
- Olsen, L., Alexander, R., Krishnan, J. and Rohner, N. (2023)
- Powers, A.K., Hyacinthe, C., Riddle, M.R., Kim, Y.K., Amaismeier, A., Thiel, K., Martineau, B., Ferrante, E., Moran, R.L., McGaugh, S.E., Boggs, T.E., Gross, J.B. and Tabin, C.J. (2023)
- Yang, Y., Xu, T., Conant, G., Kishino, H., Thorne, J.L and Ji, X. (2023)
- Sifuentes-Romero, I., Aviles, A.M., Carter, J.L., Chan-Pong, A., Clarke, A., Crotty, P., Engstrom, D., Meka, P., Perez, A., Perez, R., Phelan, C., Sharrard, T., Smirnova, M.I.,Wade, A.J. and Kowalko, J.E. (2023)
- Frøland Steindal, A., Yamamoto, Y. and Whitmore, D. (2023)
- Gross, J.B., Boggs, T.E., Rétaux, S. and Torres-Paz, J. (2023)
- Hyacinthe, C. (2023)
- Riddle, M., Nguyen, N., Nave, M., Peuß, R., Maldonado, E., Rohner, N. and Tabin, C. (2023)
- Rétaux, S. and Jeffery, W.R. (2023)
- Espinasa, L., Diamant, R., Vinepinsky, E. and Espinasa, M. (2023)
- Enriquez ,M.S., Swanson, N., Putland, R.L., Tait, T., Gluesenkamp, A.G., McGaugh, S.E. and Mensinger, A.F. (2023)
- Miranda-Gamboa, R., Espinasa, L., Verde-Ramírez, M.A., Hernández-Lozano, J., Lacaille, J.L., Espinasa, M., Ornelas-García, C.P. (2023)
- Espinasa, L. and Lewis, K.A. (2023)
- Ma, L., Yang, J.X., Lei, F.K., Xu, M.Z., Zhao, Y.H. and Jeffery, W.R. (2023)
- Perera, P.P., Guerra, D.P. and Riddle, M.R. (2023)
- Jeffery, W.R., Ma. L. and Zhao, Y.H. (2023)
- Olsen, L., Hassan, H., Keaton, S. and Rohner, N. (2023)
- Rajendran, N., Deng, F., Wang, Y., Kenzior, O., Krishnan, J., Biswas, T., Parmely, T., Rohner, N., Zhao, C. (2023)
- Stowers Institute (2023)
- Hildebrandt, M., Kotewitsch, M., Kaupp, S., Salomon, S., Schuster, S. and Machnik, P. (2024)
- Boggs, T. (2024)
- Ullah, K., Rani, R., Shaheen, F. and Tasleem, F. (2024)
- Di Lorenzo, T., Mori, M. and Simčič, T. (2024)
- Boggs, T.E. and Gross, J.B. (2024)
- Biswas, T., Hassan, H. and Rohner, N. (2024)
- Biswas T., Rajendran N., Hassan H., Li H., Zhao C. and Rohner N. (2024)
- Silva, M., Mazzoni Zerbinato Andrade Silva, D., Castro, J.P., Makunin, A.I., Barby, F.F., de Oliveira, E.H.C. (2024)
- Di Lorenzo, T., Mori, N. and Simčič, T. (2024)
- Alkafaween, E., Hassanat, A., Essa, E. and Elmougy, S. (2024)
- Swaminathan, A., Xia, F. and Rohner, N. (2024)
- Toledo Piza and 48 other authors (2024)
- Hernandez Lozano, J., Garita Alvarado, C.A., Munguia Stayer, R., Garduno Sanchez, M.A. and Ornelas Garcia, C.P. (2024)
- Manning, A.E., Grunwald, H., Moran, R., Rodriguez-Morales, R., Powers, A.K., McGaugh, S. and Kowalko, J.E. (2024)
- Accorsi, A., Guo, L., Marshall, W.F., Mommersteeg, M.T.M. and Nakajima, Y.-I. (2024)
- Espinasa, L., Tatarsky, R.L., Girard, M.K., Sandone, M., Rétaux, S. and Espinasa, J. (2024)
- Pezer, Z., Pokrovac, I. and Rohner, N. (2024)
- Lunghi E. and Bilandžija H. (2024)
- Blin, M., Valay, L., Kuratko, M., Pavie, M. and Rétaux, S. (2024)
- Turner, G.F. (2024)
- Keene, A.C., Duboue, E.R., Foulkes, N.S. and Bertolucci, C. (2024)
- Juan, T. and Ebnicher, G. (2024)
- Rodríguez-Morales, R. (2024)
- Kuball, K., Fernandes, V.F.L., Takagi, D. and Yoshizawa, M. (2024)
- Rétaux, S. and Yamamoto, K. (2024)
- Espinasa, J. and Espinasa, L. (2024)
- Brown, E.A. (2024)
- Fang, Z. (2024)
- Choy, S., Thakur, S., Polyakov, E., Abdelaziz, J., Lloyd, E., Enriquez, M., Jayan, N., Fily, Y., McGaugh, S., Keene, A.C., Kowalko, J.E. (2024)
- Pozo-Morales, M., Cobham, A.E., Centola, C., McKinney, M.C., Liu, P., Perazzolo, C., Lefort, A., Libert, F., Bai, H. Rohner, N. and Singh, S.P. (2024)
- Wiese, J., Richards, E., Kowalko, J.E. and McGaugh, S.E. (2024)
- Ng, M., Ma, L., Shi, J. and Jeffery, W.R. (2024)
- Cobham, A.E. and Rohner, N. (2024)
- Melo, B.F., Ota, R.P., Benine, R.C., Carvalho, F.R., Lima, F.C.T., Mattox, G.M.T., Souza, C.S., Faria, T.C., Reia, L., Roxo, F.F., Valdez-Moreno, M., Near, T.J., et al. (2024)
- Tidswell, B.K. (2024)
- Berning, D., Heerema, H. and Gross, J.B. (2024)
- Gallman, K., Rastogi, A., North, O., O'Gorman, M., Hutton, P., Lloyd, E., Warren, W.C., Kowalko, J.E., Duboue, E.R., Rohner, N. and Keene, A.C. (2024)
- Folgueira, M. and Clarke, J.D.W. (2024)
- Cardoso, A.L., Oliveira, J.I.N., Climaco, J.P.S., Venturelli, N.B., Moreira, D.D.N. and Martins, C. (2024)
- Pozo‐Morales, M., A. E. Cobham, C. Centola, et al. (2024)
- Di Rosa, V., Frigato, E., Negrini, P., Cristiano, W., López-Olmeda, J.F., Rétaux, S., Sánchez-Vázquez, F.J., Foulkes, N.S., Bertolucci, C. (2024)
- Policarpo, M., Legendre, L., Germon, I., Espinasa, L., Rétaux, S., Casane, D. and Lafargeas, P. (2024)
- Marketaki, S.Z., Berio, F. and Di Santo, V. (2025)
- Xia, F., Santacruz, A., Wu, D., Bertho, S., Fritz, E., Morales-Sosa, P., McKinney, S., Nowotarski, S.H. and Rohner, N. (2025)
- Knight, K. (2025)
- Choy, S., Thakur, S., Polyakov, E., Abdelaziz, J., Lloyd, E., Enriquez, M., Jayan, N., Fily, Y., McGaugh, S., Keene, A.C., Kowalko, J.E. (2025)
- Powers, A.K., Amaismeier, A., Thiel, K., Anyonge, W., McGaugh, S.E., Boggs, T.E., Tabin, C.J. and Gross, J.B. (2025)
- Legendre, L., Père, S., Rebaudo, F., Espinasa, L. and Rétaux, S. (2025)
- Biswas, T., Li, H. and Rohner, N. (2025)
- Brachmann, M.K., Costa, A.P.B., Robertson, S., Donoghue, K., Pilakouta, N., Whitehead, M., Liu, X., Kristjansson, B., Skulason, S., Selman, C. and Parsons, K. (2025)
- Fang, Z. and Atukorallaya, D. (2025)
- Huang, S., Hui, W., Kan, T., Yu, H., Liang, X., Liang, Y., Zhou, Z. and and Feng, P. (2025)
- Perera, P.P., Webster, K. and Riddle, M.R. (2025)
- Hamm, A.R. and Gross, J.B (2025)
- Cobham, A., Li, H., Acheampong, D., McKinney, C. and Rohner, N. (2025)
- Hernández-Lozano, J., Córdova-Tapia, F., Miranda-Gamboa, R., Vázquez-Cruz, MdeL., Garita-Alvarado, C., Mercado-Silva, N., Ornelas-García, C.P. (2025)
- Biswas, T., Hassan, H. and Rohner, N. (2025)
- Bauhus, M.B., Wander, L., Boy, D., Santacruz, A., Maldonado, E., Kurtz, J., Kaiser, S. and Peuß, R. (2025)
- Boggs, T.E. and Gross, J.B. (2025)
- Sekulovski, B. and Miller, N. (2025)
- Conti, F., Verges-Castillo, A., J. Sanchez-Vazquez, F.J., Lopez-Olmeda, J.F., Bertolucci, C., Munoz-Cueto, J.A. (2025)
- Roback. E.Y., Ferrufino, E., Moran, R.L., Shennard, D., Mulliniks, C., Gallop, J., Weagley, J., Miller, J., Fily, Y., Ornelas-García, C.P., Rohner, N., Kowalko, J.E. and McGaugh, S.E. (2025)
- Padmanaban, N., Ambosie. R., Choy, S., Marcus, S., Nilsson, S.R.O., Keene, A.C., Kowalko, J.E. and Duboué, E.R. (2025)
| Hubbs, C.L. and Innes, W.T. | Journal Article | 1936 | The first known blind fish of the family Characidae: A new genus from Mexico |
| Hykes, O. V. | Journal Article | 1937 | Anoptichthys jordani Hubbs and Innes |
| Innes, W.T. | Journal Article | 1937 | A cavern Characin, Anoptichthys jordani Hubbs and Innes |
| Jordan, C. B. | Journal Article | 1937 | Bringing in the new cave fish, Anoptichthys jordani Hubbs and Innes |
| Bridges, W. | Journal Article | 1940 | The blind fish of La Cueva Chica |
| Gresser, E.B. and Breder, C.M. | Journal Article | 1940 | The histology of the eye of the cave characin, Anoptichthys |
| Anonymous | Journal Article | 1940 | Expedicion para recoges peces ciegos en Mexico |
| Breder, C.M. and Gresser, E.B. | Journal Article | 1941 | Further studies on the light sensitivity and behaviour of the Mexican blind characins |
| Breder, C.M. and Gresser, E.B. | Journal Article | 1941 | Behaviour of Mexican cave characins in reference to light and cave entry |
| Breder, C.M. and Gresser, E.B. | Journal Article | 1941 | Correlations between structural eye defects and behaviour in the Mexican blind characin |
| Breder, C.M. | Journal Article | 1942 | Descriptive ecology of La Cueva Chica, with especial reference to the blind fish, Anoptichthys |
| Breder, C.M. | Journal Article | 1943 | Apparent changes in phenotypic ratios of the characins at the type locality of Anptichthys jordani Hubbs and Innes |
| Breder, C.M. | Journal Article | 1943 | Problems in the behaviour and evolution of a species of blind cave fish |
| Bridges, W. | Journal Article | 1943 | What we have learned about blind cave fishes |
| Breder, C.M. | Journal Article | 1943 | A note on erratic viciousness in Astyanax mexicanus (Phillipi) |
| Osorio Tafall, B.F. | Journal Article | 1943 | Observaciones sobra la fauna aquatica de las Cuevas de la region de Valles, San Luis Potosi (Mexico) |
| Breder, C.M. and Rasquin, P. | Journal Article | 1943 | Chemical sensory reactions in the Mexican blind characins |
| Breder, C.M. | Journal Article | 1944 | Ocular anatomy and light sensitivity studies on the blind fish Cueva de Los Sabinos, Mexico |
| Benn, J.H. | Journal Article | 1945 | Composite observations on cave life (with special reference to blind fishes) |
| Anonymous | Journal Article | 1945 | Notes on the blind cave tetra |
| Breder, C.M. | Journal Article | 1945 | Compensating reactions to the loss of the lower jaw in a cave fish |
| Rasquin, P. | Journal Article | 1946 | Progressive pigmentary regression in fishes associated with cave environments |
| Breder, C.M. and Halpern, F. | Journal Article | 1946 | Innate and aquired behaviour affecting the aggregation of fishes |
| Alvarez, J. | Journal Article | 1946 | Revision del genero Anoptichthys con descripcion de una especie nueva (Pisc., Characidae) |
| Jordan, C.B. | Journal Article | 1946 | Anoptichthys x Astyanax hybrids |
| Breder, C.M. and Rasquin, P. | Journal Article | 1947 | Comparative studies on the light sensitivity of blind characins from a series of Mexican caves |
| Breder, C.M. and Rasquin, P. | Journal Article | 1947 | Evidence for the lack of a growth principle in the optic cyst of the Mexican cave fish |
| Schlagel, S.R. and Breder, C.M. | Journal Article | 1947 | A study of the oxygen consumption of blind and eyed cave characins in light and in darkness |
| Rasquin, P. | Journal Article | 1947 | Progressive pigmentary regression in fishes associated with cave environments |
| Breder, C.M. and Roemhild, J. | Journal Article | 1947 | Comparative behaviours of various fishes under differing conditions of aggregation |
| Alvarez, J. | Journal Article | 1947 | Descripcion de Anoptichthys hubbsi caracindo ciego de la Cueva de Los Sabinos, S.L.P |
| Nigrelli R.F. | Journal Article | 1947 | Spontaneous neoplasms in fishes. III. Lymphosarcoma in Astyanax and Esox |
| Rasquin, P. | Journal Article | 1949 | The influence of light and darkness on thyroid and pituitary activity of the characin Astyanax mexicanus and its cave derivatives |
| Rasquin, P. | Journal Article | 1949 | Regeneration of the optic nerve after section with the return of vision in the characin Astyanax mexicanus |
| Rasquin, P. | Journal Article | 1949 | Spontaneous depigmentation in the catfish Ameiurus nebulosus |
| Atz, J.W. | Journal Article | 1950 | Fishes from deserts and caves |
| Breder, C.M. and Rasquin, P. | Journal Article | 1950 | A preliminary report on the role of the pineal organ in the control of pigment cells and light reactions in recent teleost fishes |
| Bolivar y Pieltain, C. | Journal Article | 1950 | Estudio de una Cirolana cavernicola nueva de la region de Valles, San Luis Potosi, Mexico |
| Villalobos, A. | Journal Article | 1951 | Un nuevo Misidaceo de las Grutas de Quintero en el estado de Tamaulipas |
| Rasquin, P. and Hafter, E. | Journal Article | 1951 | Age changes in the testis of the teleost, Astyanax mexicanus |
| Breder, C.M. and Hafter Atz., E. | Journal Article | 1952 | Conditioned restrictions of movement in fishes, fancied and real |
| Meder, E. | Journal Article | 1952 | Uber den blinden Hohlenfisch Anoptichthys jordani Hubbs und Innes. Aus der Arbeitsgemeinschaft: Naturzuchtung im VDA |
| Atz, E.H. | Journal Article | 1953 | Experimental differentiation of basophil cell types in the transitional lobe of the pituitary of a teleost fish, Astyanax mexicanus |
| Heuts, M. J. | Journal Article | 1953 | Comments on "Cave fish evolution" |
| Luling, K.H. | Journal Article | 1953 | Die Heimat und die Entdeckng unseres Blindfisches Anoptichthys jordani |
| Luling, K.H. | Journal Article | 1953 | Uber das Sehen jugendlicher Anptichthys jordani (Hubbs und Innes) |
| Luling, K.H. | Journal Article | 1953 | Uber die fortschreitende Augendegenerations des Anoptichthys jordani Hubbs und Innes (Characidae) |
| Bonet, F. | Conference Proceedings | 1953 | Datos sobre las cavernas y otros fenomenos erosivos de las calizas de La Sierra de El Abra |
| Breder, C.M. | Journal Article | 1954 | A second case of survival by a teleost without a lower jaw |
| Kuhn, O. and Kahling, G. J. | Journal Article | 1954 | Augen ruckbildung und Lichtsinn bei Anoptichthys jordani Hubbs und Innes |
| Thines, G. | Journal Article | 1954 | Etude comparative de la photosensibilite des poissons aveugles Caecobarbus geertsii Blgr et Anoptichthys jordani Hubbs et Innes |
| Luling, K.H. | Journal Article | 1954 | Untersuchungen am Blindfisch Anoptichtys jordani Hubbs und Innes (Characidae). I Einige Beobachtungen uber das Verhalten des Blindfisches Anoptichthys jordani beim Laichen |
| Luling, K.H. | Journal Article | 1954 | Untersuchungen am Blindfisch Anoptichtys jordani Hubbs und Innes (Characidae). II Beobachtungen und Experimente an Anoptichthys jordani zur Prufung der Einstellung zum Futter, zumlicht und zur Wassertubulenze |
| Stefanelli, A. | Journal Article | 1954 | It tetto ottico di Pesci ciechi cavernicoli nei riguardi del differenziamento specifico dei neuroni |
| Stefanelli, A. | Journal Article | 1954 | The differentiation of optic lobe neurones in a blind cave teleost |
| Rasquin, P. and Rosenbloom, L. | Journal Article | 1954 | Endocrine imbalance and tissue hyperplasia in teleosts maintained in darkness |
| Luling, K.H. | Journal Article | 1955 | On the subject of eye reduction found it the blind fish Anoptichthys jordani (Hubbs und Innes) from the Mexican caves |
| Bridges, W. | Book Section | 1955 | No eyes in the darkness |
| Luling, K.H. | Journal Article | 1955 | Untersuchungen am Blindfische Anoptichthys jordani, Hubbs und Innes (Characidae). III Vergleichende anatomisch-histologische studien an den Augen des Anoptichthys jordani |
| Luling, K.H. | Journal Article | 1955 | Zur Augenreduktion des aus Mexikanischen Hohlen stammenden blinden Salmlers Anoptichthys jordani (Hubbs und Innes) |
| Schutz, F. | Journal Article | 1956 | Vergleichende Untersuchungen uber die schreckreaktion bei Fischen und deren Verbreitung |
| Barr, T. C. | Journal Article | 1956 | Notes on cave blindfish |
| Sadoglu, P. | Journal Article | 1956 | A preliminary report on the genetics of the Mexican cave characin |
| Grunewald-Lowenstein, M. | Journal Article | 1956 | Influence of light and darkness on the pineal body in Astyanax mexicanus (Filippi) |
| Thines, G. and Kahling, J. | Journal Article | 1957 | Untersuchungen uber die Farbempfindlichkeit des Hohlenfisches Anoptichthys jordani (Characidae) |
| Sadoglu, P. | Journal Article | 1957 | A Mendelian gene for albinism in natural cave fish |
| Hahn, G. | Thesis | 1957 | Ferntastsinn und Stromungsssinn beim augelosen Hohlenfisch Anoptichthys jordani Hubb und Innes und das Problem der Rezeptionsorte von Lichtreizen |
| Sadoglu, P. | Journal Article | 1957 | Mendelian inheritance in the hybrids between the Mexican blind cave fishes and their overground ancestors |
| Kahling, J. | Thesis | 1957 | Progressive und regressive Augenentwicklung bei dem Hohlenfisch Anoptichthys jordani Hubbs und Innes und das Problem der Rezeptionsorte von Lichtreizenen |
| Luling, K.H. | Journal Article | 1957 | Caecobarbus geertsi Boulenger - seine Entdeckung, verbreitung und Lebensweise - und nochmals Anoptichthys jordani Hubbs und Innes |
| John, K. R. | Journal Article | 1957 | Observations on the behaviour of blind and blinded fishes |
| Stolk, A. | Journal Article | 1958 | Tumours of fishes. XXIV. Ocular melanoma in the Characid Anoptichthys jordani Hubbs et Innes |
| Cahn, P.H. | Journal Article | 1958 | Comparative optic development in Astyanax mexicanus and in two of its blind cave derivatives |
| Grobbel, G. and Hahn, G. | Journal Article | 1958 | Morphologie und Histologie der Seitenorgane des augenlosen Hohlenfisches Anoptichthys jordani im Vergleich zu anderen Teleosteern |
| Bertin, L. | Book Section | 1958 | Poissons cavernicoles |
| Pelz, H. W. | Journal Article | 1958 | Einiges uber den blinden Hohlensalmler Anoptichthys jordani Hubbs und Innes |
| Stolk, A. | Journal Article | 1959 | Experimenten met grotbewonende vissen |
| Breder, C.M. | Journal Article | 1959 | Studies on social groupings in fishes |
| Kuhn, O. | Journal Article | 1960 | Ausseroptische Lichtwerkungen bei tierischen Organismen |
| Hahn, G. | Journal Article | 1960 | Ferntastsinn und Stromungsinn beim augenlosen Hohlenfisch Anoptichthys jordani Hubbs et Innes im Vergleich zu anderen Teleostiern |
| Humbach, I. | Thesis | 1960 | Geruch und Geschmack bei den augenlosen Hohlenfischen Anopichthys jordani Hubbs und Innes und Anoptichthys hubbsi Alvarez |
| Humbach, I. | Journal Article | 1960 | Geruch und Geschmack bei den augemlosen Hohlenfischen Anoptichthys jordani Hubbs und Innes und Anoptichthys hubbsi Alvarez |
| Frank, S. | Journal Article | 1960 | Anoptichthys jordani Hubbs a Innes 1936 |
| Baslow, M. H. and Nigrelli, R. F. | Journal Article | 1961 | Muscle acetylcholinesterase levels as an index of general activity in fishes |
| Kahling, J. | Journal Article | 1961 | Untersuchungen uber den Lichtsinn und dessen Lokalisation bei dem Hohlenfisch Anoptichthys jordani Hubbs und Innes (Characidae) |
| Frank, S. | Journal Article | 1961 | A morphological study about blind cave fish, Anoptichthys jordani |
| Bath, H. | Journal Article | 1962 | Vergleichende biologische-anatomische Untersuchungen uber die Leistungsfuhigkeit der Sinnesorgane fur den Nahrungserwerbeihre gegenseitige Abhengigkeit und ihre Beziehungen zum Bau des Gehirns bei verschiedenen Knochenfischarten |
| Franck, A. | Thesis | 1962 | Mexicanische Hohlencharaciniden im Vergleich zu ihren oberirdischen Vorfahren |
| Friedman, L.R. | Journal Article | 1962 | A study of normal and malignant thymus tissue of the teleost Astyanax mexicanus in tissue culture |
| Goettert, L. | Journal Article | 1962 | Orientierungsmoglichkeiten beim augenlosen Hohlenfisch (Anoptichthys jordani) |
| Goodrick, C.L. | Journal Article | 1962 | Differential adaptation of activity in the blind cave fish, Anoptichthys jordani |
| Luling, K.H. | Journal Article | 1962 | Untersuchungen am Blindfisch Anoptichthys jordani Hubbs und Innes (Characidae). IV Bemerkungen zur Okologie und Tiergeographie |
| Pfeiffer, W. | Journal Article | 1963 | Alarm substances |
| Burgers, A.C.J., Bennink, P.J.H. and van Oordt, G.J. | Journal Article | 1963 | Investigations into the regulation of the pigmentary system in the blind Mexican cave fish Anoptichthys jordani |
| Pfeiffer, W. | Journal Article | 1963 | Vergleichende Untersuchungen uber die Schreckreaktion und den Schreckstoff der Ostariophysen |
| Berra, T.M. | Journal Article | 1963 | A study of Anoptichthys jordani |
| John, K.R. | Journal Article | 1964 | Illumination, vision, and schooling of Astyanax mexicanus (Fillipi) |
| Franck, A. | Journal Article | 1964 | Vergleichende Untersuchungen am Hohlebfisch Anoptichthys antrobius und seinem oberirdischen Vorfahren Astyanax mexicanus |
| Berra, T.M. | Journal Article | 1964 | Obstacle avoidance in the blind Mexican cave fish, characin |
| John, K.R. and Haut, M. | Journal Article | 1964 | Retinomotor cycles and correlated behaviour in the teleost Astyanax mexicanus (Fillipi) |
| Thines, G., Wolff-Van Ermengem, F., Boucquey, C. and Soffie, M. | Journal Article | 1965 | Etude comparative de l'activite du poisson cavernicole Anoptichthys antrobius Alvarez, et de son ancetre epige Astyanax mexicanus (Filippi) |
| Kosswig, C. | Journal Article | 1965 | Genetique et evolution regressive |
| Boucquey, C., Thines, G. and Van Der Borght, C. | Book Section | 1965 | Etude compartive de la capacite photopathique et de l'activite chex les poisson cavenicole Anoptichthys antrobius chez la forme epigee ancestrale Astyanax mexicanus,et chez les hybrides F1 (Astyanax x Anoptichthys) et F2 |
| Glaser, D. | Thesis | 1965 | Untersuchungen uber die absoluten Geschmacksschwellen von Fischen (Phoxinus phoxinus L., Gasterosteus aculeatus L., Hemigrammus caudovittatus Ahl, und Anoptichthys jordani Hubbs et Innes) |
| Reed, M. | Journal Article | 1966 | Raising and breeding the blind cave characin |
| Peters, N. and Peters, G. | Journal Article | 1966 | Das Auge zweier Hohlenformen von Astyanax mexicanus Filippi (Characidae, Pisces) |
| Pfeiffer, W. | Journal Article | 1966 | Die verbreitung der schreckreaktion bei kaulquappen und die herkunft des schreckstoffes |
| Pfeiffer, W. | Journal Article | 1966 | Uber die Verebung der Schreckreaktion bei Astyanax (Characidae, Pisces) |
| Carmignani, M. P. A. | Journal Article | 1966 | Distributuzione dei follicoli tiroidei lungo la regione branchiale nel teleosteo cieco Anoptichthys jordani Hubbs e Innes |
| Cavicchioli, G. and Guarnieri, P. | Journal Article | 1966 | Nota preliminari su gli organi di senso cutanei della testa e del corpo di un pesce cavernicolo Anoptichthys jordani (Cypriniformes, Characidae) |
| Thines, G., Soffie, M. and Vandenbussche, E. | Journal Article | 1966 | Analyse du comportement alimentaire du poisson cavernicole aveugle Anoptichthys Gen. et d'hybrides F1 (Astyanax x Anoptichthys) et F2 |
| Reddell, J.R. | Journal Article | 1967 | Cave biology of the Sierra de El Abra |
| Kosswig, C. | Journal Article | 1967 | Uber das Tempo evolutorischer Prozesse |
| Schemmel, C. | Journal Article | 1967 | Vergleichende Untersuchungen an den Hautsinnesorganen ober- und unterirdisch lebender Astyanax-Formen. Ein Beitrag zur evolution der Cavernicolen |
| Mattheij, J.A. and Van Oordt, P.G.W.J. | Journal Article | 1967 | The cell types in the adenohypophysis of the blind Mexican cave fish Anoptichthys jordani |
| Kuhn, O. and Strotkoetter, E. | Journal Article | 1967 | Untersuchungen von Drucken auf den tierischen Korper. 1. Druckreception bei Fischen und ihre Mitwirkung bei den Orientierung im Roum |
| Reddell, J.R. | Journal Article | 1967 | Cave biology of the Sierra de Guatemala |
| Walters, V. and Liu, R. K. | Book Section | 1967 | Hydrodynamics of navigation by fishes in terms of the mucus-water "interface" |
| Johnson, K.W. | Thesis | 1967 | Temperature responses of the Mexican blind cave-fishes of the genus Anoptichthys |
| Pfeiffer, W. | Journal Article | 1967 | Die Korrelation von Augengrosse und Mittelherngrosse bei Hybriden aus Astyanax x Anoptichthys (Characidae, Pisces) |
| Pfeiffer, W. | Journal Article | 1967 | Schreckreaction und schreckstoff-zellen bei Ostariophysi und Gonorhynchiformes |
| Pfeiffer, W. | Journal Article | 1967 | Die Korrelation von Korperlange, Augen-, Linsen, und Papillengrosse bei Hybriden aus Astyanax x Anoptichthys (Characidae, Pisces) |
| Popper, A.N. and Tavolga, W.N. | Journal Article | 1967 | Hearing thresholds in the Mexican blind cavefish |
| Sadoglu, P. | Journal Article | 1967 | The selective value of eye and pigment loss in Mexican cave fish |
| Mattheij, J.A. | Journal Article | 1968 | The ACTH cells in the adenohypophysis of the Mexican cave fish Anoptichthys jordani, as identified by metopirone (SU 4885) treatment |
| Frank, S. | Journal Article | 1968 | Der Augenlose. Der blinde Hohlensalmler kommt nicht blind zur welt |
| Carmignani, M. P. A. | Journal Article | 1968 | Morfologia ed attivita neurosecretoria del nucleo proottico e del nucleo paraipofisario nel teleosteo cieco Anoptichthys jordani Hubbs e Innes |
| Wilkens, H. | Journal Article | 1968 | Beitrag zur Degeneration des Auges bei Cavernicolen, Genzahl und Manifestationsart (Untersuchungen an mexikanischen Hohlenfischen) |
| Mattheij, J.A. | Journal Article | 1968 | The cell types in the adenohypophysis of the blind Maexican cave fish Anoptichthys jordani (Hubbs and Innes) |
| Glaser, D. | Journal Article | 1968 | Zum Verhalten blinder fische |
| Sadoglu, P. and McKee, A. | Journal Article | 1969 | A second gene that affects eye and body colour in Mexican blind cave fish |
| Mattheij, J.A. | Journal Article | 1969 | The thyrotropin secreting basophils in the adenohypophysis of Anoptichthys jordani |
| Frank, S. | Journal Article | 1969 | Blind geboren ? Entwicklung und Ruckbildung des Auges bei einem blinden Hohlensalmler |
| Mattheij, J.A. and Sprangers, J.A.P. | Journal Article | 1969 | The site of prolactin secretion in the adenohypophysis of the stenohaline teleost Anoptichthys jordani and the effects of this hormone on mucous cells |
| Campos, H. | Journal Article | 1969 | Die Geschmacksknospen im Varderdarm von Susswasserfischen, Zahl, Verteilung und Entwicklug (Phoxinus phoxinus L., Gasterosteus aculeatus L., Hemigrammus caudovittatus Ahl, Anoptichthys jordani Hubbs et Innes und Salmo gairneri Rich.) |
| John, K.R. and Kaminester, L.H. | Journal Article | 1969 | Further studies on retinomotor rhythms in the teleost Astyanax mexicanus |
| Weiss, B.A. | Journal Article | 1969 | Sonic sensitivity of blind cave fish (Anoptichthys jordani) |
| Mattheij, J.A., Sprangers, J.A.P. and Van Oordt, P.G.W.J. | Journal Article | 1969 | The site of prolactin secretion in the pituitary gland of the stenohaline teleost Anoptichthys jordani and the effects of this hormone on mucous cells |
| Morris, R.A. | Journal Article | 1969 | Blind cave fishes |
| Popper, A.N. | Thesis | 1969 | A behavioral and morphological analysis of audition in the Mexican blind cave fish, Astyanax jordani, and its eyed ancestor Astyanax mexicanus |
| Mattheij, J.A. | Journal Article | 1970 | The function of the basophilic cells in the mesoadenohypophysis of the blind cave fish, Anoptichthys jordani |
| Mattheij, J.A. | Journal Article | 1970 | The gonadotrophic cells in the adenohypophysis of the blind Mexican cave fish Anoptichthys jordani |
| Elliott, W.R. | Journal Article | 1970 | El Sótano de Soyate |
| Gertychowa, R. | Journal Article | 1970 | Studies on the ethology and space orientation of the blind cave fish Anoptichthys jordani Hubbs et Innes 1936 (Characidae) |
| Gertychowa, R. | Journal Article | 1970 | The blind fish of Cueva Chica |
| Weiss, B. and Martini, J. | Journal Article | 1970 | Lateral line sensitivity in the blind cave fish (Anoptichthys jordani) |
| Pfeiffer, W. | Journal Article | 1970 | Uber die schreckstoffzellen der Siluriformes (Ostariophysi, Pisces) |
| Popper, A.N. | Journal Article | 1970 | Auditory capacities of the Mexican blind cave fish (Astyanax jordani) and its eyed ancestor (Astyanax mexicanus) |
| Whitt, G.S. and Maeda, F.S. | Journal Article | 1970 | Lactate dehydrogenase gene function in the blind cave fish, Anoptichthys jordani, and other characins |
| Wilkens, H. | Journal Article | 1970 | Beitrag zur Degeneration des Auges bei Cavernicolen. Genzhal und Manifestationsart, Untersuchungen an Mexikanischen Hohlenfischen |
| Wilkens, H. | Journal Article | 1970 | Beitrage zur Degenerations des Melaninpigments bei cavernicolen Sippen des Astyanax mexicanus (Filippi) (Characidae, Pisces) |
| Wilkens, H. | Journal Article | 1970 | Der Bau des Auges cavernicolen Sippen von Astyanax fasciatus (Characidae, Pisces). Beitrag zur Problematik degenerativer Evolutionsprozesse |
| Wilkens, H. | Journal Article | 1971 | Genetic interpretation of regressive evolutionary processes: Studies on hybrid eyes of two Astyanax cave populations (Characidae, Pisces) |
| Reddell, J.R. and Mitchell, R.W. | Journal Article | 1971 | A checklist of the cave fauna of Mexico. I Sierra de El Abra,Tamaulipas and San Luis Potosi |
| Gertychowa, R. | Journal Article | 1971 | Heliotaktyzm mlodych rybek jaskiniowych Anoptichthys jordani Hubbs et Innes |
| Villalobos, A. | Journal Article | 1971 | Una nueva especie de Troglocubanus (Crustacea, Decapoda, Palaemonidae), de San Luis Potosí, México |
| Mollhagen, A. | Journal Article | 1971 | Checklist of bats in caves in the regions of the Sierra de Guatemala and Sierra de El Abra, northeastern México |
| Popper, A.N. | Journal Article | 1971 | The morphology of the Weberian ossicles of two species of the genus Astyanax |
| Wiley, S. and Mitchell, R.W. | Book | 1971 | A bibliography of the Mexican eyeless characin fishes of the genus Astyanax. Preliminary compilation |
| Wiley, S. and Mitchell, R.W. | Book Section | 1971 | A bibliography of the Mexican eyeless characib fishes of the genus Astyanax |
| Zaccone, G. | Journal Article | 1971 | Histomorphological and histochemical study of the ultimobranchial body of the blind teleost Anoptichthys jordani |
| Wilkens, H. and Burns, R. J. | Journal Article | 1972 | A new Anoptichthys cave population (Characidae, Pisces) |
| Wilkens, H. | Journal Article | 1972 | Uber das phylogenetische Alter von Hohlentieren. Untersuchungen über die cavernicole Süsswasserfauna Yucatans |
| Wilkens, H. | Journal Article | 1972 | Uber Praadaptionen fur das Holhlenleben, untersucht am Laichverhalten ober- und unterirdischer Populationen des Astyanax mexicanus (Pisces) |
| Wilkens, H. | Journal Article | 1972 | Zur phylogenetischen Ruckbildung des Auges Cavernicler. Untersuchungen an Anoptichthys jordani (= Astyanax mexicanus), Characidae, Pisces |
| Elliott, W.R. | Book Section | 1972 | Trip report: July - August 1969 |
| Parzefall, J. and Wilkens, H. | Journal Article | 1972 | Artbildung bei Hohlenfischen. Vergleichende Untersuchungen an zwei amerikanischen Synbranchiden |
| Zaccone, G. | Journal Article | 1972 | Comparative histochemical investigations on the mucous cells of the branchial epithelium of Mugil cephalus and Anoptichthys jordani |
| Thines, G. and Wissocq, N. | Journal Article | 1972 | Etude comparee du comportement alimentaire de deux poissons cavenicoles (Anoptichthys jordani Hubbs et Innes et Ceacobarbus geertsii Blgr) |
| Avise, J.C. and Selander, R.K. | Journal Article | 1972 | Evolutionary genetics of cave-dwelling fishes of the genus Astyanax |
| Zeitlin, S. M. | Journal Article | 1973 | Hormonal induction of ovulation and spawning in the blind cave fish, Anoptichthys jordani with the use of human chorionic gonadotropin |
| Voneida, T. J. | Journal Article | 1973 | A comparative study of retino-tectal projections in the blind cave characin, Astyanax hubbsi, and its sighted ancestor Astyanax mexicanus |
| Zeitlin, S. M. and McDevitt, D. S. | Journal Article | 1973 | Flourescent antibody study of the developing lens of the blind cave fish |
| Avise, J.C. and Kitto, G.B. | Journal Article | 1973 | Phosphoglocose isomerase gene duplication in the bony fishes:An evolutionary history |
| Schemmel, C. | Journal Article | 1973 | Les organes sensoriels cutanes du genre Astyanax (Pisces, Characidae) chez les formes occupant des biotopes souterraines |
| Mitchell, R.W. | Book Section | 1973 | Introgression between the Mexican eyeless characin fishes and their epigean ancestors |
| Mitchell, R.W. and Elliott, W.R. | Book Section | 1973 | The cave habitats of the Mexican eyeless characin fishes |
| Schmatolla, E. and Erdmann, G. | Journal Article | 1973 | Influence of retino-tectal innervation on cell proliferation and cell migration in the embryonic teleost tectum |
| Durand, J.P. | Journal Article | 1973 | Aspects ultrastructuraux des mechanismes de la rudimentation retinienne chez l'Anoptichthys adulte forme cavernicole aveugle de l'Astyanax mexicanus (Caracidae, Pisces) |
| Reddell, J.R. and Elliott, W.R. | Journal Article | 1973 | A checklist of the cave fauna of Mexico. III Additional records from the Sierra de El Abra, Tamaulipas and San Luis Potosi |
| Yasuda, K. | Journal Article | 1973 | Comparative studies on the swimming behaviour of the blind cave fish and the goldfish |
| Peters, N. and Peters, G. | Book Section | 1973 | Genetic problems in the regressive evolution of cavernicolous fish |
| Reddell, J.R. and Elliott, W.R. | Journal Article | 1973 | A checklist of the cave fauna of Mexico. V Additional records from the Sierra de Guatemala, Tamaulipas |
| Mitchell, R.W. and Elliott, W.R. | Journal Article | 1973 | The habitats of the eyeless Characin fishes of the genus Astyanax |
| Wilkens, H. | Book Section | 1973 | Phylogenetic age and degree of reduction in cave animals |
| Peters, H. and Peters, G. | Journal Article | 1973 | Problemes genetiques de l'evolution regressive des cavernicoles |
| Wilkens, H. | Journal Article | 1973 | Anciennete phylogenetique et degres de reduction chez les animaux cavernicoles |
| Thines, G. and LeGrain, J. M. | Journal Article | 1973 | Effets de la substance d'alarme sur le comportement des poissons cavernicoles Anoptichthys jordani (Characidae) et Caecobarbus geertsii (Cyprinidae) |
| Schemmel, C. | Journal Article | 1974 | Ist die cavernicole Micos-population von Astyanax mexicanus (Characidae, Pisces) hybriden ursprungs ? |
| Schemmel, C. | Journal Article | 1974 | Genetische Untersuchungen zur Evolution des Geschmacksapparates bei cavernicolen Fischen |
| Egar, M.W. | Journal Article | 1974 | An ultrastructural study of the optic nerve of the blind cave fish |
| Pfeiffer, W. | Book Section | 1974 | Pheromones in fish and amphibia |
| Sligar, C. | Journal Article | 1974 | An investigation of tectal efferents in the blind cave fish, A. hubbsi |
| Fish, J. | Journal Article | 1974 | La Sistema de Los Sabinos. Mexico's longest cave |
| Chakraborty, R. and Nei, M. | Journal Article | 1974 | Dynamics of gene differentiation between incompletely isolated populations of unequal size |
| Peters, N., Scholl, A. and Wilkens, H. | Journal Article | 1975 | Der Micos-Fisch, Hohlenfisch in statu nascendi oder Bastard ?Ein Beitrag zur Evolution der Hohlentiere |
| Omura, Y. | Journal Article | 1975 | Influence of light and darkness on the ultrastructure of the pineal organ in the blind cave fish, Astyanax mexicanus |
| Pfeiffer, W. | Journal Article | 1975 | Uber fluoreszierende Pterine aus der Haut von Cypriniformes (Pisces) und ihre Beziehung zum schreckstoff |
| Sadoglu, P. | Book Section | 1975 | Genetic paths leading to blindness in Astyanax mexicanus |
| Voneida, T. J. and Sligar, C. | Journal Article | 1976 | A comparative neuroanatomic study of retinal projections in two fishes: Astyanax hubbsi (the blind cave fish) and Astyanax mexicanus |
| Wilkens, H. | Journal Article | 1976 | Genetic and phenotypic variability in cave animals. Studies on phylogenetically young cave populations of Astyanax mexicanus (Filippi) (Characidae, Pisces) |
| Durand, J.P. | Journal Article | 1976 | Rudimentation des yeux chez les poissons et urodeles souterraines |
| Wilkens, H | Journal Article | 1976 | Genotypic and phenotypic variability in cave animals. Studies on a phylogenetically young cave population of Astyanax mexicanus (Filippi) (Characidae, Pisces) |
| Sligar, C. and Voneida, T.J. | Journal Article | 1976 | Tectal efferents in the blind cave fish Asyanax hubbsi |
| Herwig, H. J. | Journal Article | 1976 | Ultrastructural investigations on the pineal organ of a cave fish, Anoptichthys jordani and its ancestor, Astyanax mexicanus |
| Durand, J.P. | Journal Article | 1976 | Sur la structure oculaire, l'ultrastructure des muscles extrinseques, des envelopes de l'oeil et de l'epithelium pigmentaire retinien de l'Anoptichthys (forme aveugle de l'Astyanax mexicanus, Characidae, Pisces) |
| Erckens, W. and Weber, F. | Journal Article | 1976 | Rudiments of an ability for time measurement in the cavernicolous fish Anoptichthys jordani Hubbs and Innes (Pisces, Characidae) |
| Herwig, H. J. | Journal Article | 1976 | Comparative ultrastructural investigations on the pineal organ of the blind cave fish, Anoptichthys jordani and its ancestor, the eyed rive fish, Astyanax mexicanus |
| Cooper, J.E. | Journal Article | 1977 | [Review of] Mexican eyeless characin fishes of genus Astyanax: Environment, distribution and evolution (Mitchell,R.W., Russell, W.H. and Elliott, W.R.) |
| Mitchell, R.W. and Russell, W.H. | Journal Article | 1977 | The subsurface waters of the Sierra de El Abra of Mexico |
| Fish, J. | Thesis | 1977 | Karst hydrogeology and geomorphology of the Sierra de El Abra and the Valles-San Luis Potosi region, Mexico |
| Wilkens, H. | Journal Article | 1977 | Die Rudimenation des Rumpfkanals bei kavernicolen Populationen des Astyanax (Characidae, Pisces) |
| Edwards, R.J. | Journal Article | 1977 | Seasonal migrations of Astyanax mexicanus as an adaptation to novel environments |
| Mitchell, R.W., Russell, W.H. and Elliott, W.R. | Journal Article | 1977 | Mexican eyeless characin fishes, genus Astyanax: Environment,distribution and evolution |
| Yew, D. T. and Yoshihara, H. M. | Journal Article | 1977 | An ultrastructural study of the retina of the blind cave fish Astyanax hubbusi (sic) |
| Mitchell, R.W. and Elliott, W.R. | Journal Article | 1977 | The habitats of the eyeless characin fishes of the genus Astyanax |
| Pfeiffer, W. | Journal Article | 1977 | The distribution of fight reaction and alarn substance cells in fishes |
| Kirby, R.F., Thompson, K.W. and Hubbs, C. | Journal Article | 1977 | Karyotypic similarities between the Mexican and blind tetras |
| Mitchell, R.W. | Journal Article | 1977 | Introgression between the Mexican eyeless characin fishes and their epigean ancestor, Astyanax mexicanus |
| Mitchell, R.W. and Cooke, J.W. | Journal Article | 1977 | Preliminary morphometric comparisons of several populations of Mexican eyeless characin fishes of the genus Astyanax |
| Mitchell, R.W. and Russell, W.H. | Journal Article | 1977 | Stream capture in the Huastecan Province of Mexico |
| Zaccone, G. | Journal Article | 1977 | Histology, innervation, and histochemistry of the UB [Ultimobranchial] gland in the Mexican cave fish Anoptichthys jordani Hubbs et Innes (Teleostei, Characidae) |
| Birkhead, W.S. | Journal Article | 1978 | Astyanax mexicanus (Filippi) Mexican Tetra |
| Durand, J.P. | Journal Article | 1978 | Phenomenes de convergences tissulaires et cytologiques, lies aux processus degeneratifs qui affectent l'oeil chez deux teleoteens cavericoles Asytyanax (Anoptichthys) mexicanus (Characidae) et Lucifuga (Stygicola) dentatus (Ophidiidae) |
| Thines, G. and Weyers, M. | Journal Article | 1978 | Responses locomotrices du poisson cavernicole Astyanax jordani (Pisces, Characidae) a des signaux periodiques et aperiodiques de lumiere et de temperature |
| Fish, S.E. and Voneida, T.J. | Conference Paper | 1979 | Extravisual neurons in the optic tectum of a sighted and unsighted fish. 5:784 |
| Wilkens, H., Peters, H. and Schemmel, C. | Journal Article | 1979 | Gesetzmassigkeiten der regressiven Evolution |
| Woodhead, A. D. and Achey, P. M. | Journal Article | 1979 | Photoreactivating enzyme in the blind cave fish, Anoptichthys jordani |
| Pactl, J. | Journal Article | 1979 | Neue Beitrage zur Kenntnis der Apterygoten-Sammlung des Zoologischen Instituts und Zoologischen Museums der Universitat Hamburg. 6. Weitere Doppel - und Borstenschwanze (Diplura: Campodeidae; Thysanura: Lepismatidae und Nicoletiidae) |
| Durand, J.P. | Journal Article | 1979 | Aspects ultrastructuraux des mechanismes de la rudimentation retinienne chez l'Anoptichthys adult, forme cavernicole aveugle de l'Astyanax mexicanus (Characidae, Pisces) |
| Weissert, R. | Thesis | 1979 | Dressurexperimente und Verhaltensbeobachtungen zum Formunterscheidingsvermogen der blinden Hohlenfisches Anoptichthys jordani Hubbs et Innes |
| Sadoglu, P. | Journal Article | 1979 | A breeding method for blind Astyanax mexicanus based on annual spawning patterns |
| Wilkens, H. | Journal Article | 1980 | Prinzipien der Manifestation polygener Systeme |
| Schemmel, C. | Journal Article | 1980 | Studies on the genetics of feeding behaviour in the cave fish Astyanax mexicanus f. Anoptichthys. An example of apparent monofactorial inheritance by polygenes |
| Wilkens, H. | Journal Article | 1980 | Zur Problematik der Rudimentation, untersucht an der Ontogenie des Auges von Hohlenfischen (Astyanax mexicanus) |
| Weissert, R. | Book Section | 1980 | Formunterscheidung durch einer blinden Hohlenfisch (Anoptichthys jordani, Hubbs et Innes) |
| Quinn, T. P. | Journal Article | 1980 | Locomotor responses of juvenile blind cavefish, Astyanax jordani, to the odors of conspecifics |
| Campenhausen, C., Riess, I. and Weissert, R. | Journal Article | 1981 | Detection of stationary objects by blind cave fish Anoptichthys jordani (Characidae) |
| Weissert, R. and Campenhausen, C. | Journal Article | 1981 | Discrimination between stationary objects by the blind cave fish Anoptichthys jordani (Characidae) |
| Erckens, W. | Thesis | 1981 | Analyse von versuchen zur Erforschung der Grundlagen der tagesperiodishen Aktivistatsvertelung einer ober- und einer unterirdischen Populationen des Astyanax mexicanus (Characidae, Pisces) |
| Herwig, H. J. | Thesis | 1981 | The pineal organ. An ultrastructural and biochemical study on the pineal organ of Hemigrammus caudovittatus and other closely related characid fish with special reference to the Mexican blind cave fish Astyanax mexicanus |
| Erckens, W. | Journal Article | 1981 | The activity controlling time-system in epigean and hypogean populations of Astynax mexicanus (Charicidae, Pisces) |
| Dolle, A. | Thesis | 1981 | Uber Ablauf und Function des Aggressionsverhaltens von Astyanax mexicanus (Charicidae, Pisces) unter Berucksichtigung zweir Hohlenpopulationen |
| Reddell, J. R. | Book | 1981 | A review of the cavernicole fauna of Mexico, Guatemala and Belize |
| Wilkens, H. | Conference Paper | 1981 | Regressive evolution and phylogenetic age |
| Tabata, M. | Journal Article | 1982 | Persistence of pineal photosensory function in blind cave fish, Astyanax mexicanus |
| Romero, A. | Journal Article | 1982 | Troglophilic behaviour in a population of Astyanax fasciatus (Characidae): A route to cave colonisation ? |
| Jankowska, M. and Thines, G. | Journal Article | 1982 | Etude comparative de la densite de groupes de poissons cavernicoles et epigee (Characidae, Cyprinidae, Clariidae) |
| Rose, F.L. and Mitchell, R.W. | Journal Article | 1982 | Comparative lipid values of epigean and cave adapted Astyanax |
| Erckens, W. and Martin, W. | Journal Article | 1982 | Exogenous and endogenous control of swimming activity in Astyanax mexicanus (Characidae, Pisces) by direct light response and by circadian oscillator. I Analyses of the time control system of an epigean river population |
| Erckens, W. and Martin, W. | Journal Article | 1982 | Exogenous and endogenous control of swimming activity in Astyanax mexicanus (Characidae, Pisces) by direct light response and by a circadian oscillator. II Features of time controled behaviour of a cave population and their comparison to an ancestral epig |
| Lamprecht, G. and Weber, F. | Book Section | 1982 | A test for the biological significance of circadian clocks: Evolutionary regression of the time measuring ability in cavernicolous animals |
| Senkel, S. | Thesis | 1983 | Zum Schwarmverhalten von Bastarden zwischen Fluss- und Hohlenpopulationen bei Astyanax mexicanus (Pisces, Characidae) |
| Parzefall, J. | Journal Article | 1983 | Zur verebung von Verhaltensweisen bei Hohlentieren und ihren oberirdischen Verwandten |
| Zilles, K., Tillmann, B. and Bennemann, R. | Journal Article | 1983 | The development of the eye in Astyanax mexicanus (Characidae,Pisces), its blind cave derivative, Anoptichthys jordani (Characidae, Pisces, and their crossbreeds. A scanning and transmission electron microscopic study |
| Parzefall, J. | Journal Article | 1983 | Field observations in epigean and cave populations of the Mexican characid Astyanax mexcanus (Pisces, Characidae) |
| Voneida, T.J., Fish, S.E. and Cauller, T.C. | Conference Paper | 1983 | Central projections of trigeminal and lateral line nerves in the blind cave fish, Astyanax hubbsi |
| Romero, A. | Journal Article | 1983 | Introgressive hybridisation in the Astyanax fasciatus (Pisces, Characidae) population at La Cueva Chica |
| Parzefall, J. | Journal Article | 1983 | Field observations in epigean and cave populations of the Mexican characid Astyanax mexcanus (Pisces, Characidae) |
| Romero, A. | Conference Paper | 1984 | Cave colonisation by fish: The role of bat predation |
| Voneida, T. J. and Fish, S. E. | Journal Article | 1984 | Central nervous system changes related to the reduction of visual inputs in a naturally blind fish (Astyanax hubbsi) |
| Romero, A. | Journal Article | 1984 | Charles Marcus Breder, Jr. 1897-1983 |
| Fruhbeis, B. | Thesis | 1984 | Verhaltensphysiologische Untersuchungen zur Frequenz- unterscheidung und Empfindlichkeit durch das Seitenlinienorgan des blinden Hohlenfisches Anoptichthys jordani (Hubbs et Innes) |
| Mothes, P. and Jameson, R. | Journal Article | 1984 | Sotano de Vasquez |
| Romero, A. | Thesis | 1984 | Responses to light in cave and surface populations of Astyanax fasciatus (Pisces, Characidae): An evolutionary interpretation |
| Romero, A. | Journal Article | 1984 | Behaviour in an 'intermediate' population of the subterranean dwelling characid Astyanax fasciatus |
| Wilkens, H. | Journal Article | 1984 | Zur Evolution von Polygensystemen, untersucht an ober- unt unterirdischen Populationen des Astyanax mexicanus (Characidae, Pisces) |
| Schuppa, M. | Thesis | 1984 | Morphometrische und meristische Untersuchungen an verschiedenen Astyanax-populationen (Characidae) Mexicos |
| Mangold-Wernado, U. and Pfeiffer, W. | Journal Article | 1984 | Zur morphologie des Geruchsorgans von Characoidea (Ostariophysi, Pisces) |
| Hassan, E.S. | Journal Article | 1985 | Mathematical analysis of the stimulus for the lateral line organ |
| Voneida, TJ and Fish, SE | Journal Article | 1985 | An ultrastructural study of retinotectal connections and normal tectical synaptic organisation in the blind cave fish Astyanax hubbsi |
| Huppop, K. | Journal Article | 1985 | The role of metabolism in the evolution of cave animals |
| Romero, A. | Journal Article | 1985 | Cave colonisation by fish: role of bat predation |
| Burchards, H., Dolle, A. and Parzefall, J. | Journal Article | 1985 | The aggressive behaviour of an epigean population of Astyanax mexicanus (Characidae, Pisces) and some observations of three subterranean populations |
| Fricke, D. | Thesis | 1985 | Die Alarmreaktion bei Hohlen- und Flussfisch- populationen von Astyanax mexicanus (Pisces, Characidae) |
| Teyke, T. | Journal Article | 1985 | Collision with and avoidance of obstacles by blind cave fish Anoptichthys jordani (Characidae) |
| Romero, A. | Journal Article | 1985 | Morphological variation accompanying cave evolution in Astyanax fasciatus (Pisces: Characidae) |
| Romero, A. | Journal Article | 1985 | Ontogenetic change in phototactic responses of surface and cave populations of Astyanax fasciatus (Pisces, Characidae) |
| Wilkens, H. | Conference Paper | 1985 | Non-allopatric speciation in cave fishes: Studies in epigean and hypogean Astyanax populations (Characidae, Pisces) |
| Burchards, H. and Parzefall, J. | Journal Article | 1985 | Das Aggressionsverhaltens oberirdischer und unterirdischer hohlenledenden Populationen von Astyanax mexicanus (Pisces, Characidae) und ihrer Bastarde |
| Wilkens, H. | Journal Article | 1985 | The evolution of polygenic systems, studies on epigean and cave poplations of Astyanax fasciatus (Characidae, Pisces) |
| Lamprecht, G. and Weber, F. | Journal Article | 1985 | Time-keeping mechanisms and their ecological significance in cavernicolous animals |
| Hassan, E.S. | Journal Article | 1985 | Mathematical analysis of the stimulus for the lateral-line argan |
| Huppop, K. | Journal Article | 1986 | Oxygen consumption of Astyanax fasciatus (Characidae, Pisces): a comparison of epigean and hypogean populations |
| Wilkens, H. and Huppop, K. | Journal Article | 1986 | Sympatric speciation in cave fishes ? Studies on a mixed population of epi- and hypogean Astyanax (Characidae, Pisces) |
| Parzefall, J. and Senkel, S. | Journal Article | 1986 | Das schwarmverhalten bei oberirdisch und in hohlen lebenden populationen von Peocilia mexicana und Astyanax mexicanus (Peociliidae, Characidae, Pisces) |
| Parzefall, J. and Senkel, S. | Conference Paper | 1986 | Schooling behaviour in cavernicolous fish and their epigean conspecifics |
| Parzefall, J. | Journal Article | 1986 | On the heredity of behavior patterns in cave animals and their epigean relatives |
| De Fraipont, M. and Thines, G. | Journal Article | 1986 | Responses of the cave fish Astyanax mexicanus (Anoptichthys antrobius) to the odor of know or unknown conspecifics |
| Huppop, K. | Journal Article | 1986 | Comparative early life history in surface and cave fish |
| De Fraipont, M. and Thines, G. | Journal Article | 1986 | La detection chimique de l'odeur des predaeurs chex Astyanax mexixanus (formes cavernicoles et epigees) |
| Romero, A. | Journal Article | 1986 | Charles Breder and the Mexican blind cave Characid |
| Hassan, E.S. | Journal Article | 1986 | On the discrimination of spatial intervals by the blind cave fish Anoptichthys jordani |
| Wilkens, H. | Journal Article | 1986 | The evolution of polygenic systems. Studies on epigean and cave populations of Astyanax fasciatus (Characidae, Pisces) |
| Hassan, E.S. | Conference Paper | 1986 | On the discrimination of spatial intervals by the blind cave fish Anoptichthys jordani |
| Parzefall, J. | Book Section | 1986 | Behavioural ecology of cave-dwelling fishes |
| De Fraipont, M. | Journal Article | 1986 | La detection chimique de l'odeur des congeneres chex Astyanax mexicanus (forme cavernicole). Responses observees pour des cencentrations egales etablies a partir de groupes de densites differentes |
| Fraipont, M. | Journal Article | 1986 | La detection chimique chez Astyanax mexicanus (forme cavernicole) en fonction de la densite des groupes |
| De Fraipont, M. | Journal Article | 1987 | La detection chimique chez Astyanax mexicanus (forme cavernicole, Pisces, Characidae), en fonction du sexe |
| De Fraipont, M. | Journal Article | 1987 | La detection chimique chez Astyanax mexicanus (Teleostei, Characidae) (forme cavernicole) en fonction de la densite des groupes |
| Huppop, K. | Journal Article | 1987 | Food finding ability in cave fish (Astyanax fasciatus) |
| Fricke, D. | Journal Article | 1987 | Reaction to alarm substance in cave populations of Astyanax mexicanus (Characidae, Pisces) |
| Wilkens, H. | Journal Article | 1987 | Genetic analysis of evolutionary processes |
| De Fraipont, M. | Journal Article | 1988 | The responses of Astyanax mexicanus (Pisces, Characidae, epigean form) to chemical traces of conspecific groups of varying densities |
| Huppop, K. | Thesis | 1988 | Phanomene und Bedeutung der Energieersparnis bei dem Hohlenfisch Astynax fasciatus |
| Teyke, T. | Journal Article | 1988 | Flow field, swimming velocity and boundary layers: parameters which affect the stimulus for the lateral line organ in blind fish |
| Coombs, S., Janssen, J. and Webb, J. | Book Section | 1988 | Diversity of lateral line systems: evolutionary and functional considerations |
| Fricke, D. | Thesis | 1988 | Alarmreaktion und Schwarmverhalten bei ober- und unterirdisch lebenden Populationen der Art Astyanax fasciatus (Cuvier, 1819) und ihrer Bastarde (Characidae, Pisces) |
| Wilkens, H. | Journal Article | 1988 | Evolution and genetics of epigean and cave Astyanax fasciatus (Characidae, Pisces): support for the neutral mutation theory |
| Fack, H. and Wilkens, H. | Journal Article | 1989 | Eye reduction in hybrids and a naturally viable cave form Astyanax fasciatus (Characidae, Pisces) |
| Fricke, D. and Parzefall, J. | Journal Article | 1989 | Alarm reaction, aggresion and scholing in cave and river populations of Astyanax fasciatus (Pisces, Characidae) and their hybrids |
| Morris, N | Map | 1989 | Sierra De El Abra cave map portfolio |
| Huppop, K. | Journal Article | 1989 | Genetic analysis of oxygen consumption in cave and surface fish of Astyanax fasciatus (Characidea, Pisces): Further support for the neutral mutation theory |
| Teyke, T. | Journal Article | 1989 | Learning and remembering the environment in the blind cave fish Anoptichthys jordani |
| Hassan, E.S. | Book Section | 1989 | Hydrodynamic imaging of the surroundings by the lateral line of the blind cave fish Anoptichthys jordani |
| Huppop, K. | Journal Article | 1989 | Food-finding ability in cave fish (Astyanax fasciatus). (Abstract) |
| Wilkens, H. | Journal Article | 1989 | Genetic analysis of evolutionary processes. (Abstract) |
| Langecker, T. G. | Journal Article | 1989 | Studies of the light reaction of epigean and cave populations of Astyanax fasciatus (Characidea, Pisces) |
| Parzefall, J. and Fricke, D. | Journal Article | 1990 | Alarm raection and schooling in population hybrids of Astyanax (Pisces, Characidae) |
| Peters, N., Schmidt, W. and Fricke, D. | Journal Article | 1990 | Die Feinstruktur der Kolbenzellen (Schreckstoffzellen) in der Epidermis von Astyanax mexicanus Fillippi 1853 (Characidae, Pisces) und seinen Hohlen derivaten "Anoptichthys" |
| Langecker, T. G. | Journal Article | 1990 | The significance of light reactions in blind fish |
| Teyke, T. | Journal Article | 1990 | Morphological differences in neuromasts of the blind cave fish Asyanax hubbsi and the sighted river fish Astyanax mexicanus |
| Peters, N. | Journal Article | 1990 | Evolution without selection: quantitative aspects of the eye rudimenation in cave fishes |
| Langecker, T. G. | Thesis | 1990 | Der Einfluss der Lichtes bei der Evolution von Hohlenfischen |
| Yokoyama, R. and Yokoyama, S. | Journal Article | 1990 | Convergent evolution of the red- and green-like visual pigment genes in fish Astyanax fasciatus and human |
| Yokoyama, R and Yokoyama, S | Journal Article | 1990 | Isolation, DNA sequence and evolution of a color visual pigment gene of the blind cavefish Astyanax fasciatus |
| Abdel-Latif, H., Hassan, E.S. and von Campenhausen, C. | Journal Article | 1990 | Sensory performance of blind Mexican cave fish after destruction of the canal neuromasts |
| Klimpel, B. and Parzefall, J. | Journal Article | 1990 | Comparative studies of predatory behaviour in cave and river populations of Astyanax fasciatus (Characidae, Pisces) |
| Langecker, T. G. | Thesis | 1990 | Das Licht als Evolutionsfaktor bei Hohlentieren- untersucht an ober- und unterirdisch lebenden Populationen des Astyanax fasciatus (Characidae, Pisces) |
| Parzefall, J. and Fricke, D. | Journal Article | 1991 | Alarm and schooling in population hybrids of Astyanax fasciatus |
| Riedel, G. and Krug, L. | Journal Article | 1991 | Olfaktorische Regionen im Vorderhirn des blinden Hohlenfisches Asyanax hubbsi |
| Huppop, K. and Wilkens, H. | Journal Article | 1991 | Bigger eggs in subterranean Astyanax fasciatus (Characidae, Pisces) |
| Langecker, T. G., Wilkens, H. and Junge, P. | Journal Article | 1991 | Introgresve hybridisation in the Pachon cave population of Astyanax fasciatus (Teleostei, Characidae) |
| Bensouilah, M. and Denizot, J.P. | Journal Article | 1991 | Taste buds and neuromasts of Astyanax jordani: Distribution and immunochemical demonstration of co-localised Substance P and Enkephalins |
| Huppop, K. | Journal Article | 1991 | Muskulatur als Fettspeicher beim mexikanischen Hohlensalmler Astyanax fasciatus |
| Campenhausen, C. | Journal Article | 1991 | Die Bedentung der "inneren Landkarte" fur die Orientierung - Untersuchungen an blinden Hohlenfischen |
| Wilkens, H., Junge, P. and Langecker, T. G. | Journal Article | 1991 | Speciation of troglobites: Studies on the San Antonio Cave (Oaxaca, Mexico) |
| Langecker, T.G. and Longley, G. | Book Section | 1992 | Blind catfish (Trogloglanis pattersoni and Satan eurystomus) from deep artesian waters. A study on convergent adaptations to cave and deep sea biota |
| Fish, S.E. and Ghosh, P. | Conference Paper | 1992 | Occulomotor system of a blind cave fish |
| Wilkens, H. and Meyer, M. | Journal Article | 1992 | Eye formation and regression during early ontogeny in cave fishes |
| De Fraipont, M. | Journal Article | 1992 | Response d'Astyanax mexicanus aux stimulations chimiques provenant de groupes de congeneres a differents stades de developpement |
| Coombs S., Janssen J. and Montgomery J. | Book Section | 1992 | Functional and Evolutionary Implications of Peripheral Diversity in Lateral Line Systems |
| Hassan, E. S., Abdel-Latif, H. and Biebricher, R. | Journal Article | 1992 | Studies on the effects of ca++ and co++ on the swimmimg behaviour of the blind Mexican cavefish |
| Langecker, T. G. | Book Section | 1992 | Light sensitivity of cave vertebrates. Behavioural and morphological aspects |
| Zafar, N.P. and Morgan, E. | Journal Article | 1992 | Feeding entrains an endogenous rhythm of swimming activity in the blind Mexican cavefish |
| Lamprecht, G. and Weber, F. | Book Section | 1992 | Spontaneous locomotion behaviour in cavernicolous animals: The regression of the endogenous circadian system |
| Langecker, T. G. | Journal Article | 1992 | Persistence of ultrastructurally well devoloped photoreceptor cells in the pineal organ of a phylogenetically old cave dwelling population of Astyanax fasciatus Cuvier, 1819 (Teleostei, Characidae) |
| Parzefall, J. | Journal Article | 1992 | Schooloing behaviour in population hybrids of Astyanax fasciatus and Poecilia mexicana (Pisces, Characidae and Poeciliidae) |
| Wilkens, H. | Book Section | 1992 | Neutral mutations and evolutionary progress |
| Parzefall, J. | Book Section | 1992 | Behavioural aspects in animals living in caves |
| Langecker, T. G. | Journal Article | 1993 | Genetic analysis of the dorsal light reaction in epigean and cave-dwelling Astyanax fasciatus (Teleostei, Characidae) |
| Yokoyama, R. and Yokoyama, S. | Journal Article | 1993 | Molecular characterization of a blue visual pigment gene in the fish Astyanax-Fasciatus |
| Langecker, T. G., Schmale, H. and Wilkens, H. | Journal Article | 1993 | Transcription of the opsin gene in degenerate eyes of cave dwelling Astyanax fasciatus (Teleostei, Characidae) and of its conspecific epigean ancestor during early ontogeny |
| Peters, N., Schacht, V., Schmidt, W. and Wilkens, H. | Journal Article | 1993 | Gehirnproportionen und Auspragungsgrad der Sinnesorgane von Astyanax mexicanus (Pisces: Characidae). Ein Vergleich zwischen dem Flussfisch ubd seinen Hohlen-derivaten 'Anoptichthus' |
| Langecker, T. G., Schmale, H. and Wilkens, H. | Journal Article | 1993 | Strukturelle und molekulargenetische Untersuchungen zur Augenreduktion beim Hohlenfisch Astyanax fasciatus (Teleostei, Characidae) |
| Wilkens, H., Langecker, T. G. and Olcese, J. | Journal Article | 1993 | Circadian rhythms of melatonin synthesis in the pineal organ of cave-dwelling astyanax fasciatus (Teleostei: Characidae) |
| Wilkens, H. | Journal Article | 1993 | Neutral mutationen und evolutionäre Fortentwicklung |
| Hoffman, S. and Hausberg, C. | Journal Article | 1993 | The aggressive behaviour of the Micos cave population (Astyanax fasciatus, Characidae, Teleostei) after selection for functional eyes in comaprison to an epigean one |
| Parzefall, J. | Book Section | 1993 | Schooling behaviour in population hybrids of Astyanax fasciatus and Poecilia mexicana (Pisces, Characidae) |
| Parzefall, J. | Book Section | 1993 | Behavioural ecology of cave-dwelling fishes |
| Borowsky, R. | Journal Article | 1994 | Blind cave tetras of the Sierra Madre Oriental |
| Teyke, T. and Schaerer, S. | Journal Article | 1994 | Blind Mexican cave fish (Astyanax hubbsi) respond to moving visual stimuli |
| Hausberg, C. | Journal Article | 1994 | The aggressive bahaviour of the blind cave-dwelling population of the characid Astyanax fasciatus (Teleostei, Characidae) |
| Missall, J., Wilkens, H., Langecker, T. G. and Olcese, J. | Journal Article | 1994 | Studies on melatonin rhythmicity in a species of cave fish and its surface ancestor Astyanax fasciatus (Cuvier, 1819) (Characidae) |
| Missal, J, Wilkens, H, Langecker, TG and Olcese, 1994 | Journal Article | 1994 | Studies on melatonin rhythmicity in a species of cave fish and its surface ancestor Astyanax fasciatus (Cuvier, 1819) (Characidae). |
| Langecker, T. G., Neumann, B., Hausberg, C. and Parzefall, J. | Journal Article | 1995 | Evolution of the optical releasers for aggressive behaviour in cave-dwelling Astyanax fasciatus (Teleostei, Characidae) |
| Yokoyama, R, Knox, BE and Yokoyama, S | Journal Article | 1995 | Rhodopsin from the fish Astyanax: Role of Tyrosine 261 in the red shift |
| Langecker, T. G., Wilkens, H. and Schmale, H. | Journal Article | 1995 | Developmental constraints in regressive evolution: Studies of the expression of the delta-s crystallin gene in the developing lens of cave-dwelling Astaynax fasciatus (Cuvier, 1819) (Teleostei, Characidae) by in situ hybridisation |
| Montgomery, J., Coombs, S. and Halstead, M. | Journal Article | 1995 | Biology of the mechanosensory lateral line in fishes |
| Yokoyama, S., Meany, A., Wilkens, H. and Yokoyama, R. | Journal Article | 1995 | Initial mutational steps towards loss of opsin gene function in cavefish |
| Hausberg, C | Thesis | 1995 | Das Aggressionsverhalten von Astyanax fasciatus (Cuvier, 1819) Characidae Teleostei: Ontogenie, Genetik und evolution bei der epigaischen und hypogaischen Form |
| Wilkens, H. and Langecker, T. G. | Journal Article | 1996 | Vom Leben im ewigen Dunkel. Zur Evolution der Hohlenfische |
| Jeffery, W.R., Hornaday, K. and Martasian, D.P. | Journal Article | 1996 | Eye development in the cavefish Astyanax: Role of programmed cell death and the Pax-6 gene. (Abstract). |
| Borowsky, R. | Journal Article | 1996 | The Sierra de El Abra of Northeastern Mexico: blind fish in the worlds largest cave system |
| Riedel, G. | Journal Article | 1997 | The forebrain of the blind cave fish Astyanax hubbsi (Characidae). I. General anatomy of the telencephalon |
| Behrens, M., Langecker, T.G., Wilkens, H. and Schmale, H. | Journal Article | 1997 | Comparative analysis of Pax-6 sequence and expression in the eye development of the blind cavefish Astyanax fasciatus and its epigean conspecific |
| Riedel, G. and Krug, L. | Journal Article | 1997 | The forebrain of the blind cave fish Astyanax hubbsi (Characidae). II. Projections of the olfactory bulb |
| Valdez-Moreno, M.F. | Thesis | 1997 | Estudio comparativo osteologico de las formas oculada actuales del genero Astyanax en diversas cuencas de Mexico |
| Jeffery, W.R. and Martasian, D P. | Journal Article | 1997 | Evolution of eye development in the cavefish Astyanax |
| Borowsky, R. and Espinasa, L. | Journal Article | 1997 | Antiquity and origins of troglobitic Mexican tetras, Astyanax fasciatus |
| Gamboa, VJ and Ku, L | Journal Article | 1998 | Descripcion de la Cueva "Las Sardinas", Villa Luz, Mexico |
| Wilkens, H. | Journal Article | 1998 | Genetics of cave fish evolution |
| Behrens, M., Wilkens, H. and Schmale, H. | Journal Article | 1998 | Cloning of the alpha A-crystallin genes of a blind cave form and the epigean form of Astyanax mexicanus: a comparative analysis of structure, expression and evolutionary conservation |
| Buckup, P.A. | Book Section | 1998 | Relationships of the Characidiinae and phylogeny of characiform fishes (Teleostei: Ostariophysi) |
| Contreras-Balderas, S. and Lozano-Vilano, M.D.L. | Journal Article | 1998 | Problemas nomenclaturales de las formas mexicanas del genero Astyanax (Pisces: Characidae) |
| Jeffery, W.R. and Martasian, D.P. | Journal Article | 1998 | Evolution of eye regression in the cavefish Astyanax: apoptosis and the Pax-6 gene |
| Riedel, G. | Journal Article | 1998 | Long-term habituation to spatial novelty in blind cave fish (Astyanax hubbsi): role of the telencephalon and its subregions |
| Wilkens, H. | Book Section | 1998 | Genetics of cave fishes |
| Baker, C.F. and Montgomery, J. | Journal Article | 1999 | The sensory basis of rheotaxis in the blind Mexican cave fish, Astyanax fasciatus |
| Natke, C. | Thesis | 1999 | Vergleichende Untersuchungen am optischen System von Fluss- und Hohlenform des Silbersalmlers Astyanax mexicanus (Characidae, Teleostei): Faserdarstellung mit Hilfe fluoreszierender Carbocyanine |
| Estrada, M. | Thesis | 1999 | The ecology and life history of the mexican tetra, Astyanax mexicanus, (Teleostei: Characidae) in the lower Rio Grande Valley, Texas |
| Kullander, S. O. | Journal Article | 1999 | Fish species - how and why |
| Villalobos, J.L., Alvarez, F. and Iliffe, T.M. | Journal Article | 1999 | New species of troglobitic shrimps from Mexico, with the description of Troglomexicanus, new genus (Decapoda: Palaemonidae) |
| Pennisi, E. | Journal Article | 2000 | Embryonic lens prompts eye development |
| Yamamoto, Y. and Jeffery, W.R. | Journal Article | 2000 | Central role for the lens in cave fish eye degeneration |
| Huppop, K. | Book Section | 2000 | How do cave animals cope with the food scarcity in caves? |
| Ford, D.C. | Book Section | 2000 | 5.3.1. Deep phreatic caves and groundwater systems of the Sierra de El Abra, Mexico |
| Jeffery, W.R., Strickler, A.G., Guiney, S., Heyser, D.G. and Tomarev, S.I. | Journal Article | 2000 | Prox1 in eye degeneration and sensory organ compensation during development and evolution of the cavefish Astyanax |
| Espinasa, L. and Borowsky, R. | Journal Article | 2000 | Eyed cave fish in a karst window |
| Langecker, T.G. | Book Section | 2000 | The effects of continuous darkness on cave ecology and cavernicolous evolution |
| Strickler, A.G., Yamamoto, Y. and Jeffery, W.R. | Journal Article | 2001 | Early and late changes in Pax6 expression accompany eye degeneration during cavefish development |
| Avise, J.C. | Book | 2001 | Captivating life. A natutalist in the age of genetics |
| Jeffery, W.R. and Yamamoto, Y. | Journal Article | 2001 | Midline signaling by sonic hedgehog controls eye degeneration in Astyanax cavefish |
| Yokoyama, S, and Radlwimmer, F.B. | Journal Article | 2001 | The molecular genetics and evolution of red and green color vision in vertebrates |
| Yamamoto, Y. and Jeffery, W.R. | Journal Article | 2001 | [Erratum for: Central role for the lens in cavefish eye degeneration. Science 289:831-833] |
| Parzefall, J and Hausberg, C | Journal Article | 2001 | Ontogeny of the agressive behaviour in epigean and hypogean populations of Astyanax fasciatus (Characidae, teleostei) and their hybrids |
| Jeffery, W.R. | Journal Article | 2001 | Cavefish as a model system in evolutionary developmental biology |
| Montgomery, J.C., Coombs, S. and Baker, C.F. | Journal Article | 2001 | The mechanosensory lateral line system of the hypogean form of Astyanax fasciatus |
| Leclerc, P., Deharveng, L., Ng, P.K.L., Juberthie, C. and Decu, V. | Book Section | 2001 | Indonesie |
| Boudriot, F and Reutter, K | Journal Article | 2001 | Ultrastructure of the taste buds in the blind cave fish Astyanax jordani ("Anoptichthys") and the sighted river fish Astyanax mexicanus (Teleostei, Characidae) |
| Espinasa, L., Rivas-Manzano, P. and Perez, H.E. | Journal Article | 2001 | A new blind cave fish population of genus Astyanax: Geography, morphology and behaviour |
| Espinasa, L. and Borowsky, R. | Journal Article | 2001 | Origins and relationships of cave populations of the blind Mexican tetra, Astyanax fasciatus, in the Sierra de El Abra |
| Espinasa, L. | Journal Article | 2001 | Agressive behaviour and blindness: The cave of troglobitic "Astyanax" populations |
| Krejca, J. | Journal Article | 2002 | Recent field investigations of blind Astyanax |
| Wilkens, H | Journal Article | 2002 | Convergence of Astyanax cave fish evolution: Genetic evidence from eye reduction |
| Berg, A. and Watson, G.M. | Journal Article | 2002 | Rapid recovery of sensory function in blind cave fish treated with anemone repair proteins |
| Strickler, A.G., Famuditimi, K. and Jeffery, W.R. | Journal Article | 2002 | Retinal homeobox genes and the role of cell proliferation in cavefish eye degeneration |
| Dowling, T.E., Martasian, D.P. and Jeffery, W.R. | Journal Article | 2002 | Evidence for multiple genetic forms with similar eyeless phenotypes in the blind cavefish, Astyanax mexicanus |
| Romero, A., Jeffery, W.R. and Yamamoto, Y. | Journal Article | 2002 | When cave fish see the light: Reaction norm to light exposure during development in epigean, troglomorphic, and hybrids of Astyanax fasciatus (Charicidae) |
| Coombs, S, New, JG and Nelson, M | Journal Article | 2002 | Information-processing demands in electrosensory amd mechanosensory lateral line systems |
| Parzefall, J and Hausberg, C | Journal Article | 2002 | Ontogeny of the agressive behaviour in epigean and hypogean populations of Astyanax fasciatus (Characidae, Teleostei) and their hybrids |
| Borowsky, RL and Wilkens, H | Journal Article | 2002 | Mapping a cave fish genome: Polygenic systems and regressive evolution |
| Strecker, U. and Wilkens, H. | Journal Article | 2002 | Genetic divergence between cave and surface populations of Astyanax in Mexico |
| Yamamoto, Y. and Jeffery, W.R. | Journal Article | 2002 | Probing teleost eye development by lens transplantation |
| Carvalho, M.L., Oliveira, C., Navarrete, M.C., Froehlich, O. and Foresti, F. | Journal Article | 2002 | Nuclear DNA content determination in Characiformes fish (Teleostei, Ostariophysi) from the Neotropical region |
| Sarma, S.S.S., Lopez-Romulo, A. and Nandini, S. | Journal Article | 2003 | Larval feeding behaviour of blind fish Astyanax fasciatus (Characidae), black tetra Gymnocorymbus ternetzi (Characidae) and angel fish Pterophyllum scalare (Cichlidae) fed zooplankton |
| Parry, J.W.L., Peirson, S. and Wilkens, H. | Journal Article | 2003 | Multiple photopigments from the Mexican blind cavefish, Astyanax fasciatus: a microspectrophotometric study |
| Wilkens, H. and Strecker, U. | Journal Article | 2003 | Convergent evolution of the cavefish Astyanax (Characidae, teleostei): Genetic evidence from reduced eye-size and pigmentation |
| Buckup, P.A. | Book Section | 2003 | Genus Astyanax |
| Romero, A., Green, S.M., Lelonek, M.M. and Stropnicky, K.C. | Journal Article | 2003 | One eye but no vision: cavefish with induced eyes do not respond to light |
| Jeffery, W.R., Strickler, A.G. and Yamamoto, Y. | Journal Article | 2003 | To see or not to see: Evolution of eye degeneration in Mexican blind cavefish |
| Yamamoto, Y., Espinasa, L., Stock, D.W. and Jeffery, W.R. | Journal Article | 2003 | Development and evolution of craniofacial patterning is mediated by eye-dependant and -independent processes in the cavefish Astyanax |
| Reis, R.E., Kullander, S.O. and Ferraris, C.J. | Book | 2003 | Checklist of the freshwater fishes of South and Central America |
| Valdez-Moreno, M. and Contreras-Balderas, S. | Journal Article | 2003 | Skull osteology of the characid fish Astyanax mexicanus (Teleostei: Characidae) |
| Espinasa, L. and Jeffery, W.R. | Journal Article | 2003 | A troglomorphic sculpin (Pisces: Cottidae) population: Geography, morphology and conservation status |
| Espinasa, L. and Jeffery, W.R. | Journal Article | 2003 | Scientists restore eyes in blind cave fish and find virgin passage |
| Contreras-Balderas, S, Almada-Villela, P, Lozano-Vilano, M de L and Garcia-Ramirez, ME | Journal Article | 2003 | Freshwater fish at risk or extinct in Mexico |
| Strecker, U. | Journal Article | 2003 | Polymorphic microsatellites isolated from the cave fish Astyanax fasciatus |
| Porter, M.L. and Crandall, K.A. | Journal Article | 2003 | Lost along the way: the significance of evolution in reverse |
| Strecker, U., Bernatchez, L. and Wilkens, H. | Journal Article | 2003 | Genetic divergence between cave and surface populations of Astyanax in Mexico (Characidae, Teleostei) |
| Angel, T.B. | Thesis | 2003 | The neural connectivity of the Torus Semicircularis in the cave dwelling form of Astyanax fasciatus |
| Wilkens, H | Journal Article | 2004 | The Astyanax model (Teleostei): neutral mutations and directional selection |
| McCauley, D.W., Hixon, E. and Jeffery, W.R. | Journal Article | 2004 | Evolution of pigment cell regression in the cavefish Astyanax: a late step in melanogenesis |
| Yamamoto, Y | Journal Article | 2004 | Cavefish |
| Fuiman, L.A., Higgs, D.M. and Poling, K.R. | Journal Article | 2004 | Changing structure and function of the eye and lateral line system of fishes during development |
| Yamamoto, Y., Stock, D.W. and Jeffery, W.R. | Journal Article | 2004 | Hedgehog signalling controls eye degeneration in blind cavefish |
| Hooven, T.A., Yamamoto, Y. and Jeffery, W.R. | Journal Article | 2004 | Blind cavefish and heat shock protein chaperones: a novel role for hsp90alpha in lens apoptosis |
| Peters, N | Journal Article | 2004 | On the causes of brain reduction in domestic animals and cavefish |
| Soares, D., Yamamoto, Y., Strickler, A.G. and Jeffery, W.R. | Journal Article | 2004 | The lens has a specific influence on optic nerve and tectum development in the blind cavefish Astyanax |
| Burt de Perera, T. | Journal Article | 2004 | Fish can encode order in their spatial map |
| Burt de Perera, T. | Journal Article | 2004 | Spatial parameters encoded in the spatial map of the blind mexican cavefish, Astyanax fasciatus |
| Strecker, U., Faundez, V.H. and Wilkens, H. | Journal Article | 2004 | Phylogeography of surface and cave Astyanax (Teleostei) from Central and North America based on cytochrome b sequence data |
| Moreira, C.R., Bichuette, M.E., Oyakawa, O.T., de Pinna, M.C.C. and Trajano, E. | Journal Article | 2004 | Rediscovery of Stygichthys typhlops Brittan and Bohlke, 1965, an enigmatic subterranean characiform fish from Jaiba karst area, eastern Brazil |
| Jeffery, W.R. | Journal Article | 2004 | The hypogean phenotype is controlled by pleiotropic midline-signalling genes in the cavefish Astyanax mexicanus |
| Fish, J. | Journal Article | 2004 | Karst hydrology of the Sierra de El Abra |
| Burt de Perera, T and Braithwaite, VA | Journal Article | 2005 | Laterality in a non-visual sensory modality - the lateral line of fish |
| Jeffery, W.R. | Journal Article | 2005 | Evolution of eye degeneration in cavefish: The return of pleiotropy |
| Jeffery, W.R. | Journal Article | 2005 | Adaptive evolution of eye degeneration in the Mexican blind cavefish |
| Espinasa, L., Yamamoto, Y. and Jeffery, W.R. | Journal Article | 2005 | Non-optical releasers for aggressive behaviour in blind and blinded Astyanax (Teleostei: Characidae) |
| Panaram, K. and Borowsky, R.L. | Journal Article | 2005 | Gene flow and genetic variability in cave and surface populations of the Mexican tetra, Astyanax mexicanus (Teleostei: Characidae) |
| Kocher, T.D., Jeffery, W.R., Parichy, D.M., Peichel, C.L., Streelman, J.T. and Thorgaard, G.H. | Journal Article | 2005 | Special Feature—Roundtable discussion. Fish models for studying adaptive evolution and speciation |
| Tian, N.M.M.L. and Price, D.J. | Journal Article | 2005 | Why cavefish are blind |
| Trapani, J., Yamamoto, Y. amd Stock, D.W. | Journal Article | 2005 | Ontogenetic transition from unicuspid to multicuspid oral dentition in a teleost fish: Astyanax mexicanus, the Mexican tetra (Ostariophysi: Characidae) |
| Espinasa, L. and Espinasa, M. | Journal Article | 2005 | Why do cavefish lose their eyes? |
| Espinasa, L. and Espinasa, M. | Journal Article | 2005 | Why do cave fishes lose their eyes? A Darwinian mystery unfolds in the dark |
| Espinasa, L. and Jeffery, W.R. | Journal Article | 2006 | Conservation of retinal circadian rhythms during cavefish eye degeneration |
| Jeffery, W.R. | Journal Article | 2006 | Regressive Evolution of Pigmentation in the Cavefish Astyanax |
| Protas, M.E., Hersey, C., Kochanek, D., Zhou, Y., Wilkens, H., Jeffery, W.R., Zon, L.I., Borowsky, R. and Tabin, C.J. | Journal Article | 2006 | Genetic analysis of cave fish reveals molecular convergence in the evolution of albinism |
| Jeffery, W.R. | Conference Paper | 2006 | Convergence of pigment regression in cave animals: developmental, biochemical and genetic progress towards understanding evolution of the colourless phenotype |
| Franz-Odendaal, T.A. and Hall, B.K. | Journal Article | 2006 | Modularity and sense organs in the blind cavefish, Astyanax mexicanus |
| Plath, M., Rohde, M., Schroder, T., Taebel-Hellwig, A. and Schlupp, I. | Journal Article | 2006 | Female mating preferences in blind cave tetras Astyanax fasciatus (Characidae, Teleostei) |
| Reeves, R.G. and Bermingham, E. | Journal Article | 2006 | Colonization, population expansion, and lineage turnover: phylogeography of Mesoamerican characiform fish. |
| Peleshanko, S, Julian, MD, Ornatska, M, McConney, ME, LeMieux, MC, Chen, NN, Tucker, C, Yang, YC, Liu, C, Humphrey, JAC and Tsukruk, VV | Journal Article | 2007 | Hydrogel-encapsulated microfabricated haircells mimicking fish cupula neuromast |
| Di Palma, F., Kidd, C., Borowsky, R. and Kocher, T.D. | Journal Article | 2007 | Construction of bacterial artificial chromosome libraries for the Lake Malawi cichlid (Metriaclima zebra), and the blind cavefish (Astyanax mexicanus) |
| Strickler, A.G., Byerly, M.S. and Jeffery, W.R. | Journal Article | 2007 | Lens gene expression analysis reveals downregulation of the anti-apoptotic chaperone alphaA-crystallin during cavefish eye degeneration |
| Kavalco, K.F. and Almeida-Toledo, L.F. | Journal Article | 2007 | Molecular cytogenetics of blind Mexican tetra and comments on the karyotypic characteristics of genus Astyanax (Teleostei, Characidae) |
| Wilkens, H | Journal Article | 2007 | Regressive evolution: Ontogeny and genetics of cavefish eye rudimentation |
| Porter, M.L., Dittmar, K. and Perez-Losada, M. | Journal Article | 2007 | How long does evolution of the troglomorphic form take? Estimating divergence times in Astyanax mexicanus |
| Porter, M.L., Dittmar, K. and Perez-Losada, M | Book Section | 2007 | How long does evolution of the troglomorphic form take? Estimating divergence times in Astyanax mexicanus |
| Protas, M., Conrad, M., Gross, J.B., Tabin, C. and Borowsky, R.L. | Journal Article | 2007 | Regressive evolution in the Mexican cave tetra, Astyanax mexicanus |
| Holbrook, R., Bomphrey, R., Walker, S., Taylor, G., Thomas, A. and Burt de Perera, T. | Journal Article | 2007 | Swimming performance of a subcarangiform, the blind mexican cave fish (Astyanax fasciatus) |
| Menuet, A., Alunni, A., Joly, J.S., Jeffery, W.R. and Retaux, S. | Journal Article | 2007 | Expanded expression of sonic hedgehog in Asyanax cavefish: multiple consequences on forebrain development and evolution |
| Alunni, A., Menuet, A., Candal, E., Penigault, J.B., Jeffery, W.R. and Retaux, S. | Journal Article | 2007 | Developmental mechanisms for retinal degeneration in the blind cavefish Astyanax mexicanus |
| Gregson, J.N.S. and Burt de Perera, T. | Journal Article | 2007 | Shoaling in eyed and blind morphs of the characin Astyanax fasciatus under light and dark conditions |
| Strickler, A.G., Yamamoto, Y. and Jeffery, W.R. | Journal Article | 2007 | The lens controls cell survival in the retina: Evidence from the blind cavefish Astyanax |
| Moreira, C.R. | Thesis | 2007 | Relações filogenéticas na ordem Characiformes (Teleostei: Ostariophysi) |
| Borowsky, R.L. | Journal Article | 2008 | Restoring sight in blind cavefish |
| Borowsky, R.L. | Journal Article | 2008 | Are cave populations evolutionary dead ends? |
| Windsor, S.P. | Thesis | 2008 | Hydrodynamic imaging by blind Mexican cavefish |
| Ornelas-Garcia, C.P., Dominguez-Dominguez, O. and Doadrio, I. | Journal Article | 2008 | Evolutionary history of the fish genus Astyanax Baird and Girard (1854) (Actinopterygii, Characidae) in Mesoamerica reveals multiple morphological homoplasies |
| Borowsky, R.L. | Journal Article | 2008 | Determining the sex of adult Astyanax mexicanus |
| Borowsky, R.L. | Journal Article | 2008 | Breeding Astyanax mexicanus through natural spawning |
| Borowsky, R.L. | Journal Article | 2008 | In vitro fertilisation of Astyanax mexicanus |
| Borowsky, R.L. | Journal Article | 2008 | Handling Astyanax mexicanus eggs and fry |
| Wilkens, H. | Journal Article | 2008 | Genes, modules and the evolution of troglobites |
| Espinasa, M. and Espinasa, L. | Journal Article | 2008 | Losing sight of regressive evolution |
| Borowsky, R. | Journal Article | 2008 | Astyanax mexicanus, the blind Mexican cave fish: a model for studies in development and morphology |
| Yoshizawa, M. and Jeffery, W.R. | Journal Article | 2008 | Shadow response in the blind cavefish Astyanax reveals conservation of a functional pineal eye |
| Niven, J.E. | Journal Article | 2008 | Evolution: Convergent eye losses in fishy circumstances |
| Salin, K., Voituron, Y., Colson, C. and Hervant, F. | Journal Article | 2008 | To colonise subterranean environments without starvation tolerance capacity: the case of fish Astyanax mexicanus |
| Protas, M., Tabansky, I., Conrad, M., Gross, J.B., Vidal, O., Tabin, C. and Borowsky, R.L. | Journal Article | 2008 | Multi-trait evolution in a cave fish, Astyanax mexicanus |
| Yong, E. | Journal Article | 2008 | Blind fish see shadows |
| Holbrook, R., Bomphrey, R., Walker, S., Taylor, G., Thomas, A. and Burt de Perera, T. | Journal Article | 2008 | A comparative analysis of the normal swimming performance of two subcarangiforms, blind Mexican cave fish (Astyanax fasciatus) and goldfish (Carassius auratus) |
| Gross, J.B., Protas, M., Conrad, M., Scheid, P.E., Vidal, O., Jeffery, W.R., Tabin, C. and others | Journal Article | 2008 | Synteny and candidate gene prediction using an anchored linkage map of Astyanax mexicanus |
| Holbrook, R.I. and Burt de Perera, T. | Journal Article | 2008 | Three-dimensional orientation in fish |
| Retaux, S, Pottin, K and Alunni, A | Journal Article | 2008 | Shh and forebrain evolution in the blind cavefish Astyanax mexicanus |
| Aw, J.M., Holbrook, R.I., Burt de Perera, T. and Kacelnik, A. | Journal Article | 2008 | Apparent irrationality and state-dependant learning in fish |
| Windsor, S., Mallinson, G. and Montgomery, J. | Journal Article | 2008 | Hydrodynamic imaging by blind Mexican cave fish (Astyanax fasciatus) |
| Strecker, U. | Journal Article | 2008 | Introgression in Astyanax fasciatus cave populations |
| Windsor, S., Tan, D. and Montgomery, J. | Journal Article | 2008 | Swimming kinematics and hydrodynamic imaging in the blind Mexican cave fish (Astyanax fasciatus) |
| Jeffery, W.R. | Journal Article | 2008 | Developmental relationships between eye regression and constructive traits in Astyanax cavefish |
| Moreira, C.R. and Trajano, E. | Book Section | 2008 | Stygichthys typhlops Brittan and Bohlke, 1965 |
| Jeffery, W.R. | Journal Article | 2008 | Emerging model systems in evo-devo: cavefish and microevolution of development |
| Holbrook, R. and Burt de Perera, T. | Journal Article | 2009 | Separate encoding of vertical and horizontal components of space during orientation in fish |
| Albertson, R.C., Cresko, W., Detrich, H.W. and Postlethwait, J.H. | Journal Article | 2009 | Evolutionary mutant models for human disease |
| Patton, P., Coombs, S. and Windsor, S. | Conference Paper | 2009 | Active wall following in the Mexican blind cavefish (Astyanax sp.) |
| Jeffery, W.R. | Conference Paper | 2009 | AIM 2009: The first international Astyanax meeting |
| Jeffery, W.R. | Journal Article | 2009 | Regresive evolution in Astyanax cavefish |
| Maher, B. | Journal Article | 2009 | Evolution: biology's next top model |
| Streets, A and Soares, D | Conference Paper | 2009 | Changes in sensory strategies during development of cavefish |
| Porter, M.L., Jeffery, W.R. and Dittmar, K. | Conference Paper | 2009 | Estimating divergence times in Astyanax mexicanus |
| Jeffery, W.R. | Journal Article | 2009 | Evolution of eye degeneration in cavefish |
| Esquivel Bobadilla, S., Borowsky, R.L., Espinosa Perez, H. and Garcia de Leon, F.J. | Conference Paper | 2009 | Microsatellite polymorphisms of Astyanax mexicanus at Mexican Atlantic slope |
| Borowsky, R.L. | Book Section | 2009 | Emerging model organisms Astyanax mexicanus, the blind Mexican cave fish: A model for studies in development and morphology |
| Jeffery, W.R. | Journal Article | 2009 | Evolution and development in the cavefish Astyanax |
| McHenry, M., Feitl, K. and Cardenas, G. | Conference Paper | 2009 | Do blind Astyanax have highly sensitive lateral line receptors? |
| Culver, D.C. and Pipan, T. | Book | 2009 | The biology of caves and other subterranean habitats |
| Reynoso, V.H., Paredes-Leon, R. and Arroyo Lambaer, D. | Conference Paper | 2009 | Conservation isues in the blind cave fishes of the genus Astyanax in northeastern Mexico |
| Ornelas-Garcia, C.P., Dominguez-Dominguez, O. and Doadrio, I. | Conference Paper | 2009 | Phylogeny and biogeography of the genus Astyanax in Mesoamerica |
| Strickler, A.G. and Jeffery, W.R. | Journal Article | 2009 | Differentially expressed genes identified by cross-species microarray in the blind cavefish Astyanax |
| Yoshizawa, M., Soares, D., Goricki, S., Ashida, G. and Jeffery, W.R. | Conference Paper | 2009 | Evolution of adaptive behaviours in the cavefish Astyanax |
| De Carvalho, P.A., Paschoalini, A.L., Santos, G.B., Rizzo, E. and Bazzoli, N. | Journal Article | 2009 | Reproductive biology of Astyanax fasciatus (Pisces: Characiformes) in a reservoir in southeastern Brazil. |
| Cronk, Q.C.B. | Journal Article | 2009 | Evolution in reverse gear: the molecular basis of loss and reversal |
| Yamamoto, Y., Byerly, M.S., Jackman, W.R. and Jeffery, W.R. | Journal Article | 2009 | Pleiotropic functions of embryonic sonic hedgehog expression link jaw and tastebud amplification with eye loss during cavefish evolution |
| Varatharasan, N., Croll, R. and Franz-Odendaal, T. | Journal Article | 2009 | Taste bud development and patterning in sighted and blind morphs of Astyanax mexicanus. |
| Valdez-Moreno, M., Ivanova, N.V., Elias-Gutierrez, M., Contreras-Balderas, S. and Herbert, P.D.N. | Journal Article | 2009 | Probing diversity in freshwater fishes from Mexico and Guatemala with DNA barcodes |
| Sharma, S., Coombs, S., Patton, P. and Burt de Perera, T. | Journal Article | 2009 | The function of wall-following behaviours in the Mexican blind cavefish and a sighted relative, the Mexican tetra (Astyanax) |
| Franz-Odendaal, T., Varatharasan, N. and Croll, R.P. | Journal Article | 2009 | The development of taste buds in two morphs of Astyanax mexicanus |
| Leclercq, E., Taylor, J.F. and Migaud, H. | Journal Article | 2009 | Morphological skin colour changes in teleosts |
| McConney, M.E., Chen, N.N., Lu, D., Hu, H., Coombs, S., Liu, C. and Tsukruk, V.V. | Journal Article | 2009 | Biologically inspired design of hydrogel-capped hair sensors for enhanced underwater flow detection |
| Coombs, S. and Patton, P. | Conference Paper | 2009 | Short-range, sensorimotor adaptations for aquiring spatial knowledge |
| Kowalko, J., Gross, J.B., Protas, M., Borowsky, R.L. and Tabin, C. | Conference Paper | 2009 | Genetic approaches to studying morphological and behavioural traits in Astyanax mexicanus |
| Dufton, M. and Franz-Odendaal, T.A. | Conference Paper | 2009 | Sensory structures and developmental modules within Astyanax |
| Ren, W.Y. and Yamamoto, Y. | Conference Paper | 2009 | How cavefish lost their eyes. Midline signalling and eye development |
| Pottin, K and Retaux, S | Conference Paper | 2009 | Interactions between signalling centres for anterior neural plate patterning in Astyanax |
| Gross, J.B., Borowsky, R. and Tabin, C. | Journal Article | 2009 | A novel role for Mc1r in the parallel evolution of depigmentation in independant populations of the cavefish Astyanax mexicanus |
| Sutherland, L., Holbrook, R.I. and Burt de Perera, T. | Journal Article | 2009 | Sensory system affects orientational strategy in a short-range spatial task in blind and eyed morphs of the fish, Astyanax fasciatus |
| Guibal, C., Yamamoto, Y., Reynoso, V.H. and Whitmore, D. | Conference Paper | 2009 | Biological clocks in Astyanax mexicanus |
| Retaux, S., Alunni, A., Menuet, A. and Pottin, K. | Conference Paper | 2009 | Why do cavefish first develop eyes? A forebrain deveopment hypothesis |
| Aw, J.M., Holbrook, R.I., Burt de Perera, T. and Kacelnik, A. | Journal Article | 2009 | State-dependant valuation learning in fish: Banded tetras prefer stimuli associated with greater past deprivation |
| Espinasa, L. and Giribet, G. | Journal Article | 2009 | Living in the dark—species delimitation based on combined molecular and morphological evidence in the nicoletiid genus Texoreddellia Wygodzinsky, 1973 (Hexapoda: Zygentoma: Nicoletiidae) in Texas and México |
| Whitmore, D., Guibal, C., Tamai, K. and Yamamoto, Y. | Conference Paper | 2009 | Circadian clocks and light sensitivity in Astyanax cell lines |
| Windsor, S. | Conference Paper | 2009 | Hydrodynamic imaging in blind Mexican cave fish |
| Holbrook, R. and Burt de Perera, T. | Conference Paper | 2009 | Spatial orientation of two morphs of Astyanax fasciatus |
| Jeffery, W.R. | Conference Paper | 2010 | Pleiotropic tradeoffs between constructive and regressive traits during troglomorphic evolution |
| Moreira, C.R., Bichuette, M.E., Oyakawa, O.T., de Pinna, M.C.C. and Trajano, E. | Journal Article | 2010 | Rediscovery and redescription of the unusual subterranean characiform Stygichthys typhlops, with notes on its life history |
| Taylor, G.K., Holbrook, R. and Burt de Perera, T. | Journal Article | 2010 | Fractional rate of change of swim-bladder volume is reliably related to absolute depth during vertical displacements in teleost fish |
| Patton, P., Windsor, S. and Coombs, S. | Journal Article | 2010 | Active wall following by Mexican blind cavefish (Astyanax mexicanus) |
| Windsor, S.P., Norris, S.E., Cameron, S.M., Mallinson, G.D. and Montgomery, J.C. | Journal Article | 2010 | The flow fields involved in hydrodynamic imaging by blind Mexican cave fish (Astyanax fasciatus). Part II: gliding parallel to a wall. |
| Mirande, J.M. | Journal Article | 2010 | Phylogeny of the family Characidae (Teleostei: Characiformes): from characters to taxonomy |
| Van Trump, W.J., Coombs, S., Duncan, K. and McHenry, M. | Conference Paper | 2010 | Gentamicin disrupts both receptor classes in the lateral line system |
| Windsor, S.P., Norris, S.E., Cameron, S.M., Mallinson, G.D. and Montgomery, J.C. | Journal Article | 2010 | The flow fields involved in hydrodynamic imaging by blind Mexican cave fish (Astyanax fasciatus). Part I: open water and heading towards a wall. |
| Jeffery, W.R. | Journal Article | 2010 | Pleiotropy and eye degeneration in cavefish |
| Juan, C., Guzik, M.T., Jaume, D. and Cooper, S.J.B. | Journal Article | 2010 | Evolution in caves: Darwin’s ‘wrecks of ancient life’ in the molecular era |
| Jeffery, W.R. and Strickler, A.G. | Book Section | 2010 | Development as an evolutionary process in Astyanax cavefish |
| Wilkens, H | Journal Article | 2010 | Genes, modules and the evolution of cave fish |
| Yoshizawa, M., Goricki, S., Soares, D. and Jeffery, W.R. | Journal Article | 2010 | Evolution of a behavioural shift mediated by superficial neuromasts helps cavefish find food in darkness |
| Pottin, K., Hyacinthe, C., and Retaux, S. | Journal Article | 2010 | Conservation, development, and function of a cement gland-like structure in the fish Astyanax mexicanus |
| Salin, K., Voituron, Y., Mourin, J. and Hervant, F. | Journal Article | 2010 | Cave colonization without fasting capacities: an example with the fish Astyanax fasciatus mexicanus |
| Coombs. S. | Conference Paper | 2010 | Active flow-sensing for spatial exploration and navigation |
| Gross, J.B. and Tabin, C.J. | Book Section | 2010 | Evolutionary genetics of pigmentation loss |
| Van Trump, W.J., Coombs, S., Duncan, K. and McHenry, M.J. | Journal Article | 2010 | Gentamicin is ototoxic to all hair cells in the fish lateral line system |
| Retaux, S, Pottin, K and Hyacinthe, C | Journal Article | 2011 | The nature and function of the Astyanax casquette |
| Jeffery, W.R. and Bilandzija, H. | Conference Paper | 2011 | Convergent evolution of albinism in cave animals: A defect in the first step of melanin biosynthesis in troglomorphic fishes and insects |
| Espinasa, L. | Conference Paper | 2011 | Promoting undergraduate research in large classrooms: Guerrero cave Astyanax - Old or young colonisation? |
| Retaux, S | Conference Paper | 2011 | Projection of a movie promoting Astyanax as a model system |
| Holbrook, R. and Burt de Perera, T. | Journal Article | 2011 | Three-dimensional spatial cognition: information in the vertical dimension overrides information from the horizontal |
| Pottin, K., Hinaux, H. and Retaux, S. | Journal Article | 2011 | Restoring eye size in Astyanax mexicanus blind cavefish embryos through modulation of the Shh and Fgf8 forebrain organising centres |
| Kish, P.E., Bohnsack, B.L., Gallina, D., Kasprick, D.S. and Kahana, A. | Journal Article | 2011 | The eye as an organizer of craniofacial development |
| Gatenby, R.A., Gillies, R.J. and Brown, J.S. | Journal Article | 2011 | Of cancer and cave fish |
| Holbrook, R. and Burt de Perera, T. | Journal Article | 2011 | Fish navigation in the vertical dimesion: can fish use hydrostatic pressure to determine depth? |
| Kasumyan, A.O. | Journal Article | 2011 | Tactile reception and behavior of fish |
| Windsor, S., Paris, J. and Burt de Perera, T. | Journal Article | 2011 | No role for direct touch using the pectoral fins, as an information gathering strategy in blind fish |
| Ornelas-Garcia, C.P., Pedraza-Lara, C.P., Bastir, M. and Doadrio, I. | Conference Paper | 2011 | Parallel adaptive divergence in a characid fish genus Asyanax Baird and Girard (1854) from Catemaco Lake (Mexico) |
| Ma, L and Jeffery, WR | Conference Paper | 2011 | The function of alphaA-Crystallin in lens degeneration in Astyanax mexicanus cavefish |
| Avise, J.C. | Conference Paper | 2011 | What's so special about cave fish |
| Wilkens, H. | Journal Article | 2011 | Variability and loss of functionless traits in cave animals. Reply to Jeffery (2010) |
| Gallo, N.D. and Jeffery, W.R. | Conference Paper | 2011 | Variance of space dependant growth in Astyanax mexicanus |
| Duboue, E.R. and Borowsky, R.L. | Conference Paper | 2011 | Evolutionary convergence on sleep loss in cavefish populations |
| Duboue, E.R., Keene, A. and Borowsky, R. | Journal Article | 2011 | Evolutionary convergence on sleep loss in cavefish populations |
| Strickler, A.G. and Soares, D. | Journal Article | 2011 | Comparative genetics of the central nervous system in epidean and hypogean Astyanax mexicanus |
| Vazquez-Echeverria, C., Guibal, C., Reynoso, V.H., Carrillo-Rosas, S., Ramos-Balderas, J.L. and Maldonado, E. | Conference Paper | 2011 | Cloning ear development related genes in Astyanax mexicanus |
| Yoshizawa M. and Jeffery, W.R. | Journal Article | 2011 | Evolutionary tuning of an adaptive behavior requires enhancement of the neuromast sensory system |
| Yamamoto, Y. and Jeffery, W.R. | Book Section | 2011 | Blind cavefish |
| Kowalko, J., Rohner, N., Borowsky, R.L. and Tabin, C. | Conference Paper | 2011 | Cave fish as a model to elucidate the genetic basis of the evolution of behaviour |
| Lechner, W. and Ladich, F. | Journal Article | 2011 | How do albino fish hear? |
| Yoshizawa, M. and Jeffery, W.R. | Conference Paper | 2011 | Evolution of a behaviour mediated by the lateral line system adapts Astyanax to life in darkness |
| Salin, K., Voituron, Y., Mourin, J. and Hervant, F. | Conference Paper | 2011 | Cave colonisation without fasting capacities: An example with the fish Astyanax fasciatus mexicanus |
| Elipot, Y., Callebert, J., Hinaux, H. and Retaux, S. | Conference Paper | 2011 | Linking evolution of the aggressive behaviour and the serotogenic system in Astyanax mexicanus surface fish and blind cavefish populations |
| Cavallari, N., Frigato, E., Vallone, D., Froehlich, N., Lopez-Olmeda, J.F., Foa, A., Berti, R., Sanchez-Vazquez, F.J., Bertolucci, C. and Foulkes, N.S. | Journal Article | 2011 | A blind circadian clock in cavefish reveals that opsins mediate peripheral clock photoreception |
| Oliveira, C., Avelino, G.S., Abe, K.T., Mariguela, T.C., Benine, R.C., Orti, G., Vari, R.P. and Correa e Castro, R.M. | Journal Article | 2011 | Phylogenetic relationships within the speciose family Characidae (Teleostei: Ostariophysi: Characiformes) based on multilocus analysis and extensive ingroup sampling |
| Pottin, K., Hinaux, H., Elipot, Y., Chaloub, H., Pere, S. and Retaux, S. | Conference Paper | 2011 | A developmental staging table for Astyanax mexicanus |
| Bradic, M., Beerli, P. and Borowsky, R.L. | Journal Article | 2011 | Population genetic evidence for convergance and parallelism in the Mexican blind cavefish (Astyanax mexicanus) |
| Tan, D., Patton, P. and Coombs, S. | Journal Article | 2011 | Do blind cavefish have behavioural specializations for active flow-sensing? |
| Legendre, L., Elipot, Y., Hinaux, H., Pere, S., Sohm, F. and Retaux, S. | Conference Paper | 2011 | I-Scel mediated transgenesis in Astyanax model |
| Schobert, C.S., Stiassny, M.L.J., Jeffery, W.D. and Dubielzig, R.R. | Journal Article | 2011 | Comparative ocular anatomy In troglomorphic fish: Sensory compensation for reduced vision |
| Hinaux, H., Pottin, K., Chalhoub, H., Pere, S., Elipot, Y., Legendre, L. and Retaux, S. | Journal Article | 2011 | A developmental staging table for Astyanax mexicanus surface fish and Pachon cavefish |
| Hausdorf, B., Wilkens, H. and Strecker, U. | Journal Article | 2011 | Population genetic patterns revealed by microsatellite data challemge the mitochondrial DNA based taxonomy of Astyanax in Mexico (Characidae, Teleostei) |
| Esquivel-Bobadilla, S | Thesis | 2011 | Análisis genético de Astyanax mexicanus (Characidae, Teleostei, Pisces) de la vertiente atlántica de México usando microsatélites. |
| Hinaux, H and Retaux, S | Journal Article | 2011 | Placode development in Astyanax mexicanus blind cavefish and sighted surface fish |
| Hinaux, H., Fartman, B., DaSilva, C., Noirot, C. and Jeffery, W.R. | Conference Paper | 2011 | Astyanax mexicanus cavefish and surface fish cDNA libraries |
| Strecker, U., Hausdorf, B. and Wilkens, H. | Journal Article | 2012 | Parallel speciation in Astyanax cave fish (Teleostei) in Northern Mexico |
| Yoshizawa, M., Yamamoto, Y., O'Quin, K.E. and Jeffery, W.R. | Journal Article | 2012 | Evolution of an adaptive behavior and its sensory receptors promotes eye regression in blind cavefish |
| Dufton, M., Hall, B.K. and Franz-Odendaal, T. | Journal Article | 2012 | Early lens ablation causes dramatic long-term effects on the shape of bones in the craniofacial skeleton of Astyanax mexicanus |
| Porter, M.L., Blasic, J.R., Bok, M.J., Cameron, E.G., Pringle, T., Cronin, T.W. and Robinson, P.R. | Journal Article | 2012 | Shedding new light on opsin evolution |
| Jeffery, W.R. | Book Section | 2012 | Astyanax mexicanus: A model organism for evolution and adaptation |
| Pipan, T. and Culver, D.C. | Journal Article | 2012 | Convergence and divergence in the subterranean realm: a reassessment. |
| Huppop, K. | Book Section | 2012 | Adaptation to low food |
| Gallo, N.D. and Jeffery, W.R. | Journal Article | 2012 | Evolution of space dependent growth in the teleost Astyanax mexicanus |
| Yoshizawa, M., Ashida, G. and Jeffery, W.R. | Journal Article | 2012 | Parental genetic effects in a cavefish adaptive behavior explain disparity between nuclear and mitochondrial DNA |
| Hoke, K., Schwartz, A. and Soares, D. | Journal Article | 2012 | Evolution of the fast start response in the cavefish Astyanax mexicanus |
| Idda, M.L., Bertolucci, C., Vallone, D., Gothilf, Y., Sánchez-Vázquez, F.J. and Foulkes N.S. | Book Section | 2012 | Circadian clocks: lessons from fish |
| Gross, J. B. and Wilkens, H. | Journal Article | 2012 | Evolution of albinism in a captive population of cavefish |
| Duboue, E.R., Borowsky, R.L. and Keene, A.C. | Journal Article | 2012 | Beta-adrenergic signaling regulates evolutionarily derived sleep loss in the Mexican cavefish |
| Duboue, E.R. and Borowsky, R. | Journal Article | 2012 | Altered rest-activity patterns evolve via circadian independent mechanisms in cave adapted balitorid loaches |
| Wilkens, H. | Book Section | 2012 | Neutral mutations |
| Mogdans, J. and Bleckmann, H. | Journal Article | 2012 | Coping with flow: behavior, neurophysiology and modeling of the fish lateral line system |
| Trontelj, P. | Book Section | 2012 | Natural selection |
| Hervant, F. | Book Section | 2012 | Starvation in subterranean species versus surface-dwelling species: Crustaceans, Fish, and Salamanders |
| Gross, J.B. | Journal Article | 2012 | The complex origin of Astyanax cavefish |
| Protas, M. and Jeffery, W.R. | Journal Article | 2012 | Evolution and development in cave animals: from fish to crustaceans |
| Franck, D | Journal Article | 2012 | Curt Kosswig. Ein Forscherleben zwischen Bosporus und Elbe |
| Bradic, M., Beerli, P., Garcia-de Leon, F.J., Esquivel-Bobadilla, S. and Borowsky, R.L. | Journal Article | 2012 | Gene flow and population structure in the Mexican blind cavefish complex (Astyanax mexicanus) |
| Blin, M., Bibliowicz, Y. and Rétaux, S. | Conference Paper | 2013 | Development of the olfactory system in Astyanax cavefish and surface fish |
| Stahl, A.L., Stahl, B.A., Buschbeck, E. and Gross, J.B. | Conference Paper | 2013 | An evaluation of eyelessness in cave dwelling Astyanax mexicanus using RNA-seq technology |
| Hinaux, H., Poulain, J., Da Silva, C., Noirot, C., Jeffery, W. R., Casane, D. and Retaux, S. | Journal Article | 2013 | De novo sequencing of Astyanax mexicanus surface fish and Pachon cavefish transcriptomes reveals enrichment of mutations in cavefish putative eye genes |
| OʼQuin, K.E., Yoshizawa, M., Doshi, P. and Jeffery, W.R. | Conference Paper | 2013 | Quantitative genetic analysis of retinal degeneration in the blind cavefish A. mexicanus |
| Wall, A. and Volkoff, H. | Journal Article | 2013 | Effects of fasting and feeding on the brain mRNA expressions of orexin, tyrosine hydroxylase (TH), PYY and CCK in the Mexican blind cavefish (Astyanax fasciatus mexicanus) |
| Bibliowicz, J., Elipot, Y., Blin, M. and Rétaux, S. | Conference Paper | 2013 | Olfactory evolution in cave-dwelling Astyanax mexicanus |
| Bilandzija, H., Ma, L., Parkhurst, A. and Jeffery, W.R. | Journal Article | 2013 | A potential benefit of albinism in Astyanax cavefish: Downregulation of the oca2 gene increases Tyrosine and catecholamine levels as an alternative to melanin synthesis |
| Kowalko, J.E., Rohner, N., Rompani, S.B., Peterson, B.K., Linden, T.A., Yoshizawa, M., Kay, E.H., Weber, J., Hoekstra, H.E., Jeffery, W.R., Borowsky, R. and Tabin, C.J.. | Journal Article | 2013 | Genetic analysis of the loss of schooling behavior in cavefish reveals both sight-dependent and independent mechanisms. |
| Coghill, L.M. | Thesis | 2013 | Statistical and comparative phylogeography of Mexican freshwater taxa in extreme aquatic environments |
| Friedrich, M. | Journal Article | 2013 | Biological clocks and visual systems in cave-adapted animals at the dawn of speleogenomics |
| Espinasa, L. | Conference Paper | 2013 | Pardigm shifts and pendulum swings regarding the origin of Astyanax cavefish: What about geology? |
| Pennisi, E. | Journal Article | 2013 | Cavefish study supports controversial evolutionary mechanism |
| Borowsky, R. and Cohen, D. | Journal Article | 2013 | Genomic consequences of ecological speciation in Astyanax cavefish |
| Espinasa, L. and Jeffery, W. | Conference Paper | 2013 | Caballo Moro breaks Dollo's law: Recuperation of vision in a blind cavefish population |
| Atukorala, A.D.S., Hammer, C., Dufton, M. and Franz-Odendaal, T.A. | Journal Article | 2013 | Adaptive evolution of the lower jaw dentition in Mexican tetra (Astyanax mexicanus) |
| Bobadilla, S.E., García de León, F.J. and Borowsky, R. | Conference Paper | 2013 | Genetic structure of Astyanax mexicanus at Mexican Atlantic slope |
| Retaux, S. and Casane, D. | Journal Article | 2013 | Evolution of eye development in the darkness of caves: adaptation, drift, or both? |
| Van Trump, W.J. and McHenry, M.J. | Journal Article | 2013 | The lateral line system is not necessary for rheotaxis in the Mexican blind avefish (Astyanax fasciatus) |
| Borowsky, R. | Journal Article | 2013 | Eye regression in blind Astyanax cavefish may facilitate the evolution of an adaptive behavior and its sensory receptors |
| Retaux, S., Elipot, Y., Prunier, L. Hinaux, H. and Blin, M. | Conference Paper | 2013 | Feed or fight: Delelopmental origin of a behavioural shift in blind cavefish |
| Borowsky, R. | Conference Paper | 2013 | Divergence and speciation in Astyanax of the Sierra de El Abra |
| Vazquez, A.O.S., Bárcenas-Luna, R. and Arellano-Carbajal, F. | Conference Paper | 2013 | Comparative phylogenies of monogenean parasites and their host Astyanax mexicanus |
| Gunter, H. and Meyer, A. | Journal Article | 2013 | Trade-offs in cavefish sensory capacity |
| Retaux, S. and Elipot, Y. | Journal Article | 2013 | Feed or fight: A behavioral shift in blind cavefish |
| Beale, A., Guibal, C., Tamai, T.K., Klotz, L., Cowen, S., Peyric, E., Reynoso, V.H., Yamamoto, Y. and Whitmore, D. | Journal Article | 2013 | Circadian rhythms in Mexican blind cavefish Astyanax mexicanus in the lab and in the field |
| Stemmer, M., Schuhmacher, L.-N., Foulkes, N.S., Bertolucci, C. and Wittbrodt, J. | Conference Paper | 2013 | Unravelling continuous eye growth in teleosts by studying blind cavefish |
| Garcia-Gonzalez, O.M. and Arellano-Carbajal, F. | Conference Paper | 2013 | Isolation and characterization of V1r pheromone receptor gene in cave and surface variants of Astyanax mexicanus |
| Beale, A. | Thesis | 2013 | The circadian clock of the Mexican blind cavefish, Astyanax mexicanus |
| Ma, L. and Jeffery, W. | Conference Paper | 2013 | Role of aA-crystallin in Asyanax cavefish eye degeneration |
| Rétaux, S., Bourrat, F., Joly, J.-S. and Hinaux, H., | Book Section | 2013 | Perspectives in evo-devo of the vertebrate brain |
| Rodrigues, F.R. | Thesis | 2013 | Comparison of brain and cranial nerve morphology between eyed surface fish and blind cave fish of the species Astyanax mexicanus. |
| Gross, J. B., Krutzler, A.J. and Espinasa, L. | Conference Paper | 2013 | An analysis of structural mutations in the gene Mc1r in surface and Granadas cave-dwelling Astanax aeneus |
| Pazza, R., Bertollo, L.A.C., de Almeida-Toledo, L.F. and Kavalco, K.F. | Conference Paper | 2013 | Molecular systematics of the genus Astyanax - Starter edition |
| Jeffery, W., Doshi, P., Yoshizawa, M. and O’Quin, K.E. | Conference Paper | 2013 | Development and genetics of the Astyanax sclera: An optic tissue organised by the lens |
| Gross, J.B., Krutzler, A.J. and Bruns, L.E. | Journal Article | 2013 | Genetic analysis of dermal bone fragmentation in a natural model system |
| Joachim, B.L., Riesch R., and Jeffery, W.R., and Schlupp, I. | Journal Article | 2013 | Pignent cell retention in cavernicolous populations of Poecilia mexicana (Poeciliidae) |
| Gross, J.B. and Wilkens, H. | Journal Article | 2013 | Albinism in phylogenetically and geographically distinct populations of Astyanax cavefish arises through the same loss-of-function Oca2 allele |
| Gross, J. B. and Matthews, M | Conference Paper | 2013 | An analysis of gene expression level changes across development in surface and cave dwelling fish |
| Elliott, W.R. | Conference Paper | 2013 | Astyanax: Looking back 45 years |
| Borowsky, R.L. | Journal Article | 2013 | Evolution of an adaptive behaviour and its sensory receptors facilitates eye regression in blind cavefish |
| Hinaux, H., Poulain, J., Da Silva, C., Noirot, C., Jeffery, W.R., Casane, D. and Rétaux, S. | Conference Paper | 2013 | Transcriptome analysis in Astyanax mexicanus blind cavefish and sighted surface fish |
| Kowalko, J.E., Rohner, N., Rompani, S.B., Peterson, B.K., Linden, T.A., Yoshizawa, M., Kay, E.H., Weber, J., Hoekstra, H.E., Jeffery, W.R., Borowsky, R. and Tabin, C.J. | Journal Article | 2013 | Loss of schooling behavior in cavefish through sight-dependent and sight-independent mechanisms |
| Keene, A. | Conference Paper | 2013 | Metabolic regulation of sleep in A. mexicanus |
| O'Quin, K.E., Yoshizawa, M., Doshi, P. and Jeffery, W R. | Journal Article | 2013 | Quantitative genetic analysis of retinal degeneration in the blind cavefish Astyanax mexicanus |
| Bibliowicz, J., Alie, A., Espinasa, L., Yoshizawa, M., Blin, M., Hinaux, H., Legendre, L., Pere, S. and Retaux, S. | Journal Article | 2013 | Differences in chemosensory response between eyed and eyeless Astyanax mexicanus of the Rio Subterraneo cave |
| Krutzler, A.J., Bruns, L.E. and Gross, J.B. | Conference Paper | 2013 | Fragmentation, fussion and asymmetry in the craniofacial skeleton of Astyanax mexicanus |
| Rohner, N., Jarosz, D.F., Kowalko, J.E., Yoshizawa, M., Jeffery, W.R., Borowsky, R.L., Lindquist, S. and Tabin, C.J. | Journal Article | 2013 | Cryptic variation in morphological evolution: HSP90 as a capacitor for loss of eyes in cavefish |
| Yoshizawa, M., O'Quin, K.E. and Jeffery, W.R. | Journal Article | 2013 | Evolution of an adaptive behavior and its sensory receptors promotes eye regression in blind cavefish: response to Borowsky (2013) |
| Gross, J.B., Furterer, A., Carlson, B.M. and Stahl, B.A. | Journal Article | 2013 | An integrated transcriptome-wide analysis of cave and surface dwelling Astyanax mexicanus |
| Legendre, L., Elipot, Y., Hinaux, H., Pere, S., Sohm, F. and Retaux, S. | Conference Paper | 2013 | Transgenesis methods in Astyanax |
| Furterer, A., Carlson, B.M., Stahl, B.A. and Gross, J.B. | Conference Paper | 2013 | An intergrated transcriptone-wide analysisn of cave and surface dwelling Astyanax mexicanus |
| Rohner, N., Jarosz, D.F., Taipale, M., Kowalko, J., Yoshizawa, M., Jeffery, W.R., Borowsky, R.L., Lindquist, S. and Tabin, C.J. | Conference Paper | 2013 | HSP90 as a capacitor for the evolution of eye loss in fish |
| Yoshizawa, M., O'Quin, K.E., Ashida, G. and Jeffery, W.R. | Conference Paper | 2013 | Adaptive changes in vibration attraction behaviour and its sensory receptors promote eye degeneration and disparity between the nuclear and mitochondrial genomes in Pachon cavefish |
| Elipot, Y., Hinaux, H., Callebert, J. and Retaux, S. | Journal Article | 2013 | Evolutionary shift from fighting to foraging in blind cavefish through changes in the serotonin network |
| Bradic, M., Teotonio, H. and Borowsky, R.L. | Journal Article | 2013 | The population genomics of repeated evolution in the blind cavefish Astyanax mexicanus |
| Stahl, B.A. and Gross, J.B. | Conference Paper | 2013 | Pigmentation loss in cave animals: A high-resolution study of destructive genetic mutations |
| Kowalko, J., Rohner, N., Linden, T.A., Rompani S.B., Warren, W.C., Borowsky, R., Tabin, C.J., Jeffery, W.R. and Yoshizawa M. | Journal Article | 2013 | Convergence in feeding posture occurs through different loci in independently evolved cave populations of Astyanax mexicanus. |
| Yoshizawa, M., O’Quin, K.E., and Jeffery, W.R. | Journal Article | 2013 | QTL clustering as a mechanism for rapid multi-trait evolution |
| Yoshizawa, M., Jeffery, W.R., van Netten, S.M. and McHenry, M.J. | Journal Article | 2014 | The sensitivity of lateral line receptors and their role in the behavior of Mexican blind cavefish (Astyanax mexicanus) |
| Elipot, Y., Hinaux, H., Callebert, J., Launay, J.-M., Blin, M. and Retaux, S. | Journal Article | 2014 | A mutation in the enzyme monoamine oxidase explains part of the Astyanax cavefish behavioural syndrome |
| Bleckmann, H., Mogdans, J. and Coombs, S.L. | Book | 2014 | Flow sensing in air and water. Behavioral, neural and engineering principles of operation |
| Holzman, R., Perkol-Finkel, S. and Zilman, G. | Journal Article | 2014 | Mexican blind cavefish use mouth suction to detect obstacles |
| Trajano, E. and Moreira, C.R. | Journal Article | 2014 | Stygichthys typhlops Brittan and Bohlke, 1965 (Teleostei: Characiformes), a phreatobic fish from eastern Brazil: Comments on Sampaio et al. (2012) |
| Espinasa, L., Bibliowicz, J., Jeffery, W. R. and Retaux, S. | Journal Article | 2014 | Enhanced prey capture skills in Astyanax cavefish larvae are independent from eye loss |
| Atukorallaya, D. and Franz-Odendaal, T. | Journal Article | 2014 | Astyanax mexicanus-A novel model of tooth shape formation and regeneration |
| Espinasa, L., Bartolo, N.D. and Newkirk, C.E. | Journal Article | 2014 | DNA sequences of troglobitic nicoletiid insects supports Sierra de El Abra and the Sierra de Guatemala as a single biogeographic area: Implications for Astyanax |
| Espinasa, L., Centone, D.M. and Gross, J.B. | Journal Article | 2014 | A contemporary analysis of a loss-of-function oculocutaneous albinism type II (Oca2) allele within the Rio Subterraneo Astyanax cavefish population |
| Atukorala, A.D.S. and Franz-Odendaal, T.A. | Journal Article | 2014 | Spatial and temporal events in tooth development of Astyanax mexicanus |
| McGaugh, S.E., Gross, J.B., Aken, B., Blin, M., Borowsky, R., Chalopin, D., Hinaux, H., Jeffery, W.R., Keene, A., Ma, L., Minx, P., Murphy, D., O'Quin, K.E., Retaux, S., Rohner, N., Searl, S.M., Stahl, B.A., Tabin, C., Volff, J.N., Yoshizawa M, Warren WC. | Journal Article | 2014 | The cavefish genome reveals candidate genes for eye loss |
| Moran, D., Softley, R. and Warrant, E.J. | Journal Article | 2014 | Eyeless Mexican cavefish save energy by eliminating the circadian rhythm in metabolism |
| Windsor, S.P. | Book Section | 2014 | Hydrodynamic imaging by blind Mexican cavefish |
| Penney, C.C. and Volkoff, H. | Journal Article | 2014 | Peripheral injections of cholecystokinin, apelin, ghrelin and orexin in cavefish (Astyanax fasciatus mexicanus): Effects on feeding and on the brain expression levels of tyrosine hydroxylase, mechanistic target of rapamycin and appetite-related hormones |
| Ma, L., Parkhurst, A. and Jeffery, W.R. | Journal Article | 2014 | The role of a lens survival pathway including sox2 and [alpha]A-crystallin in the evolution of cavefish eye degeneration |
| Elliott, W.R. | Journal Article | 2014 | Sotano de Yerbaniz |
| Coghill, L.M., Hulsey, C.D., Chaves-Campos, J., de Leon, F.J.G. and Johnson, S.G. | Journal Article | 2014 | Next generation phylogeography of cave and surface Astyanax mexicanus |
| Soares, D. Niemiller, M.L. and Higgs, D. | Journal Article | 2014 | Review article. Hearing and acoustic communication in cavefishes |
| Schartl, M. | Journal Article | 2014 | Beyond the zebrafish: Diverse fish species for modeling human disease. |
| Montgomery, J., Bleckmann, H. and Coombs, S. | Book Section | 2014 | Sensory ecology and neuroethology of the lateral line |
| Gross, J.B., Krutzler, A.J. and Carlson, B.M. | Journal Article | 2014 | Complex craniofacial changes in blind cave-dwelling fish are mediated by genetically symmetric and asymmeric loci |
| Elipot, Y., Legendre, L., Pere, S., Sohm, F. and Retaux, S. | Journal Article | 2014 | Astyanax transgenesis and husbandry: How cavefish enters the laboratory |
| Coombs, S., Bleckmann, H., Fay, R.R. and Popper, A.N. | Book | 2014 | The Lateral Line System |
| Palacios-Vargas, J.G., Juberthie, C. and Reddell, J. | Book | 2014-2015 | Encyclopaedia Biospeologica Mexico |
| Caballero-Hernandez, O., Hernandez-Patricio, M., Sigala-Regalado, I., Morales-Malacara, J.B. and Miranda-Anaya, M. | Journal Article | 2015 | Circadian rhythms and photic entrainment of swimming activity in cave-dwelling fish Astyanax mexicanus (Actinopterygii: Characidae), from El Sotano La Tinaja, San Luis Potosi, Mexico |
| Yoshizawa, M. and Macaspac, C. | Conference Paper | 2015 | How to build DIY water-flow system that successfully makes a cavefish spawn |
| Riddle, M. and Tobin, C.J. | Conference Paper | 2015 | Astyanax mexicanus as a model to study metabolism and the evolution of the digestive system |
| Stahl, B.A. and Gross, J.B. | Journal Article | 2015 | Alterations in Mc1r gene expression are associated with regressive pigmentation in Astyanax cavefish |
| Kulpa, M., Bak-Coleman, J. and Coombs, S. | Journal Article | 2015 | The lateral line is necessary for blind cavefish rheotaxis in non-uniform flow |
| O’Quin, K.E., Doshi, P., Lyon, A., Hoenemeyer, E., Yoshizawa, M. and Jeffery, W,R. | Journal Article | 2015 | Complex evolutionary and genetic patterns characterize the loss of scleral ossification in the Blind cavefish, Astyanax mexicanus |
| Bradic, M, Rohner, N, Tabin, C and Borowsky, R. | Conference Paper | 2015 | Caves with eyed and eyeless populations of Astyanax |
| Yoshizawa, M. | Journal Article | 2015 | Behaviors of cavefish offer insight into developmental evolution. |
| Stahl, B.A., Ma, L. and Gross, J.B. | Conference Paper | 2015 | High resolution genomic mapping reveals genes contributing to complex melaophore variation in Astyanax mexicanus cavefish |
| Reyes, W.D. | Thesis | 2015 | Effects of temperature and water flow on morphology of Astyanax mexicanus (Teleostei: Characidae): |
| Bilandzija, H. | Conference Paper | 2015 | The loss of body pigmentation has evolved independantly in multiple animal taxa that have successfully colonised subterranean habitats |
| Rohner, N., Aspiras, A., Borowsky, R. and Tabin, C. | Conference Paper | 2015 | Hungry, fat and healthy - Studying the physiological basis of cave adaptation |
| Soares, D. | Conference Paper | 2015 | Functional imaging of circuits commonly associated with vision processing in the Astyanax blind cavefish |
| Kasumyan, A. O. and Marusov, E. A. | Journal Article | 2015 | Chemoorientation in the feeding behavior of the blind Mexican cavefish Astyanax fasciatus (Characidae, Teleostei) |
| Powers, A.K., Sung, J.Y.T. and Gross, J.B. | Conference Paper | 2015 | Investigating a potential relationship between constructive trait evolution and aberant cranial phenotypes in Asyanax cavefish |
| Keene, A. | Conference Paper | 2015 | Convergent evolution of sleep loss |
| Silva, D. M. Z. A., Utsunomia, R., Pansonato-Alves, J.C., Oliveira, C. and Foresti, F. | Journal Article | 2015 | Chromosomal mapping of repetitive DNA sequences in five species of Astyanax (Characiformes, Characidae) reveals independent location of U1 and U2 snRNA sites and association of U1 snRNA and 5S rDNA |
| Aspiras, A.C., Rohner, N., Martineau, B., Borowsky, R.L. and Tabin, C.J. | Conference Paper | 2015 | Melanocortin 4 receptor mutations contribute to the adaptation of cavefish to nutrient-poor conditions |
| Robinson, B.G., Jaggard, J.B., Yoshizawa, M. and Keene, A.C. | Conference Paper | 2015 | The evolution of sleep loss in relation to metabolic processes in Mexican cavefish |
| Dufton, M. and Franz-Odendaal, T.A. | Journal Article | 2015 | Morphological diversity in the orbital bones of two teleosts with experimental and natural variation in eye size |
| McGaugh, S. E., Weagley, J., Jeffery, W.R., Yoshizawa, M., O'Quin, K., Espinasa, L., Rohner, N., Borowsky, R. and Warren, W. | Conference Paper | 2015 | Population genomics of cavefish |
| Moran, D., Softley, R. and Warrant, E.J. | Journal Article | 2015 | The energetic cost of vision and the evolution of eyeless Mexican cavefish |
| Aspiras A.C., Rohner N., Martineau B., Borowsky R.L. and Tabin C.J. | Journal Article | 2015 | Melanocortin 4 receptor mutations contribute to the adaptation of cavefish to nutrient-poor conditions |
| Hinaux, H., Blin, M., Fumey, J., Legendre, L., Heuze, A., Casane, D. and Retaux, S. | Journal Article | 2015 | Lens defects in Astyanax mexicanus cavefish: Evolution of crystallins and a role for alphaA-crystallin |
| Yoshizawa, M., Robinson, B.G., Duboue, E.R., Masek, P., Jaggard, J.B., O'Quin, K.E., Borowsky, R.L., Jeffery, W.R. and Keene, A.C. | Journal Article | 2015 | Distinct genetic architecture underlies the emergence of sleep loss and prey-seeking behavior in the Mexican cavefish |
| Hinaux, H., Recher, G., Blin, M., Devos, L., Alié, A. and Rétaux, S. | Conference Paper | 2015 | Sensory evolution in blind cavefish is driven bt early embryonic events during gasrulation and neurulation |
| Stemmer, M., Schuhmacher, L.-N., Foulkes, N.S., Bertolucci, C. and Wittbrodt, J. | Journal Article | 2015 | Cavefish eye loss in response to an early block in retinal differentiation progression |
| Fumey, J., Hinaux, H., Noirot, C., Rétaux S. and Casane, D. | Conference Paper | 2015 | Evidence of late Pleistocene origin of Astyanax mexicanus cavefish |
| Hernandez Mena, D., Santacruz Vázquez, A.O., Ornelas García, P., Mendoza Garfias, B., Ponce de León, G.P. | Conference Paper | 2015 | Endohelminth parasites of the genus Astyanax through its geographical distribution in Mexico |
| Jaggard, J.B., Robinson, B., Oh, I., Masek, P., Yoshizawa, M. and Keene, A. | Conference Paper | 2015 | Distinct neural mechanisms underlie the convergent evolution of sleep loss in Astyanax |
| Ma, L., Carlson, B., Stahl, B., Powers, M. and Gross, J.B. | Conference Paper | 2015 | Candidate genes analysis for pigment development in Astyanax cavefish |
| Niven, J.E. | Journal Article | 2015 | Neural evolution: Costing the benefits of eye loss |
| Ma, L., Jeffery, W.R., Essner, J.J. and Kowalko, J.E. | Journal Article | 2015 | Genome editing using TALENs in blind Mexican cavefish, Astyanax mexicanus |
| Hammer, C., Atukorala, A.D.S. and Franz‐Odendaal, T. | Journal Article | 2015 | Dentition influences shape of oral jaws in fish |
| Tabin, J., Rohner, N., Haro, A., Kowalko, J., Martineau, B., Borowsky, R. and Tabin, C. | Conference Paper | 2015 | The evolution of temperature in Astyanax |
| Ma, L. and Jeffery, W.R. | Conference Paper | 2015 | Molecular analysis of an A. mexicanus eye QTL reveals a potential role of Cystathionine B-synthase A in cavefish eye degeration |
| Gross, J.B., Meyer, B. and Perkins, M. | Journal Article | 2015 | The rise of Astyanax cavefish |
| Devos, L, Alie, A. and Retaux, S. | Conference Paper | 2015 | Developmental evolution of the basal forebrain in cavefish |
| Sumi, K., Asaoka, R., Nakae, M. and Sasaki, K. | Journal Article | 2015 | Innervation of the lateral line system in the blind cavefish Astyanax mexicanus (Characidae) and comparisons with the eyed surface-dwelling form |
| Garcia, O.M., Santacruz, A., Reynoso, V.H. and Maldonado, E. | Conference Paper | 2015 | Spatial memory experiments in Astyanax mexicabus |
| Carlson, B.M., Onusko, S.W. and Gross, J.B. | Journal Article | 2015 | A high-density linkage map for Astyanax mexicanus Using genotyping-by-sequencing technology |
| Carlson, B.M. and Gross, J.B. | Conference Paper | 2016 | The unusual suspects: Genetic analysis reveals candidate genes potentially underlying altered activity profiles in the blind Mexican tetra, Astyanax mexicanus |
| Hinaux, H., Retaux, S. and Elipot, Y. | Book Section | 2016 | Social behavior and aggressiveness in Astyanax |
| Yamamoto, Y. | Book Section | 2016 | Molecular Mechanisms of Eye Degeneration in Cavefish |
| Volkoff, H. | Book Section | 2016 | Feeding behavior, starvation response, and endocrine regulation of feeding in Mexican blind cavefish (Astyanax fasciatus mexicanus) |
| Hinaux, H., Devos, L., Blin, M., Elipot, Y., Bibliowicz, J., Alei, A. and Retaux, S. | Journal Article | 2016 | Sensory evolution in blind cavefish is driven by early embryonic events during gastrulation and neurulation. |
| Kowalko, J.E., Ma, L. and Jeffery, W.R. | Journal Article | 2016 | Genome editing in Astyanax mexicanus using transcription activator-like effector nucleases (TALENs) |
| Gross, J.B. | Book Section | 2016 | Convergence and parallelism in Astyanax cave-dwelling fish |
| Ma, L., Stahl, B., Adams, H. and Gross, J. | Conference Paper | 2016 | Molecular analysis of melanophore lineage genes in cavefish depigmentation |
| Yoshizawa, M. | Book Section | 2016 | The evolution of sensory adaptation in Astyanax mexicanus |
| Pennisi, E. | Journal Article | 2016 | Antisocial cave fish may hold clues to schizophrenia, autism |
| Gross, J.B., Stahl, B.A., Powers, A.K. and Carlson, B.M. | Journal Article | 2016 | Natural bone fragmentation in the blind cave-dwelling fish, Astyanax mexicanus: candidate gene identification through integrative comparative genomics |
| Jeffery, W.R., Ma, L., Parkhurst, A. and Bilandzija, H. | Book Section | 2016 | Pigment regression and albinism in Astyanax cavefish |
| Rohner, N. | Book Section | 2016 | Selection through standing genetic variation |
| Burt de Perera, T., Holbrook, R. and Davis, V. | Journal Article | 2016 | The representation of three-dimensional space in fish |
| Pennisi, E | Journal Article | 2016 | Blind cave fish may provide insights into human health |
| Borowsky, R. | Book Section | 2016 | Regressive evolution: Testing hypotheses of selection and drift |
| Bilandžija, H. | Conference Proceedings | 2016 | Evolution of melanin pigment regression in cave animals |
| Casane, D. and Retaux, S. | Journal Article | 2016 | Evolutionary genetics of the cavefish Astyanax mexicanus |
| Gross, J.B. and Powers, A.K. | Book Section | 2016 | The evolution of the cavefish craniofacial complex |
| Yoshizawa, M., Settle, A., Macaspac, C., Fernandes, V. Yoshida, M. and Keene, A. | Conference Paper | 2016 | Adaptation through changes of behavioral and morphological traits in Mexican Cavefish |
| Aspiras, A., Tabin, C. and Rohner, N. | Conference Paper | 2016 | Astyanax mexicanus as a natural model for metabolic adaptation |
| Bilandžija, H. | Conference Proceedings | 2016 | Adaptive differences between dopamine-related locomotor activity in cave and surface dwelling Astyanax mexicanus |
| Gross, J.B., Gangidine, A and Powers, A.K. | Journal Article | 2016 | Asymmetric facial bone fragmentation mirrors asymmetric distribution of cranial neuromasts in blind Mexican cavefish |
| Espinasa, L. and Espinasa, M. | Book Section | 2016 | Hydrogeology of Caves in the Sierra de El Abra Region |
| Shoen, E., Jeffery, W. and Bilandžija, H. | Conference Paper | 2016 | Adaptive differences between dopamine-related locomotor activity in cave and surface dwelling Astyanax mexicanus |
| Gross, J.B., Powers, A.K., Davis, E.M. and Kaplan, S.A. | Journal Article | 2016 | A pleiotropic interaction between vision loss and hypermelanism in Astyanax mexicanus cave x surface hybrids |
| Powers, A. Davis, E., Kaplan, S. and Gross, J. | Conference Paper | 2016 | The evolution of craniofacial shape change in the blind Mexican Cavefish. |
| Atukorala, A.D.S. and Franz-Odendaal, T.A. | Book Section | 2016 | Evolution and Development of the Cavefish Oral Jaws: Adaptations for Feeding |
| Beale, A.D. and Whitmore, D. | Book Section | 2016 | Daily rhythms in a timeless environment: Circadian clocks in Astyanax mexicanus |
| Hollifield, B., Bilandžija, H. and Jeffery, W. | Conference Paper | 2016 | Understanding the colonization of caves: Effects of constant darkness on the surface form of Astyanax mexicanus |
| O'Quin, K. and McGaugh, S.E. | Book Section | 2016 | Mapping the genetic basis of troglomorphy in Astyanax: How far we have come and where do we go from here? |
| Beale, A., Whitmore, D. and Moran, D. | Journal Article | 2016 | Life in a dark biosphere: A review of circadian physiology in "arrhythmic" environments |
| Schmitter-Soto, J.J. | Journal Article | 2016 | A phylogeny of Astyanax (Characiformes: Characidae) in Central and North America |
| Martin, L., Bilandžija, H., Soueidan, S. and Jeffery, W. | Conference Paper | 2016 | The relationship between differential anesthesia tolerance and melanin pigment development in cave-adapted and surface Astyanax mexicanus |
| Foulkes, N.S., Whitmore, D., Vallone, D and Bertolucci, C. | Journal Article | 2016 | Studying the evolution of the vertebrate circadian clock: The power of fish as compataive models |
| Keene, A.C., Yoshizawa, M. and McGaugh, S.E. | Book | 2016 | Biology and Evolution of the Mexican Cavefish |
| Piscor, D. and Parise-Maltempi, P. P. | Journal Article | 2016 | Chromosomal mapping of H3 histone and 5S rRNA genes in eight species of Astyanax (Pisces, Characiformes) with different diploid numbers: syntenic conservation of repetitive genes |
| Wilkens, H | Journal Article | 2016 | Genetics and hybridization in surface and cave Astyanax (Teleostei): a comparison of regressive and constructive traits |
| Soares, D., Niemiller, M.L. and Higgs, D.M. | Journal Article | 2016 | Hearing in Cavefishes |
| Santacruz, A., Garcia, O.M.., Tinoco-Cuellar, M., Rangel-Huerta, E. and Maldonado, E. | Book Section | 2016 | Spatial mapping in perpetual darkness: EvoDevo of behavior in Astyanax mexicanus cavefish |
| Duboue, E.R. and Keene, A.C. | Book Section | 2016 | Investigating the evolution of sleep in the Mexican cavefish |
| Piscor, D., Centofante, L and Parise-Maltempi, P. P. | Journal Article | 2016 | Highly similar morphologies between chromosomes bearing U2 snRNA gene clusters in the group Astyanax Baird and Girard 1854 (Characiformes: Characidae): An evolutionary approach in species with 2n=36, 46, 48 and 50 |
| Elliott, W.R. | Book Section | 2016 | Cave biodiversity and ecology of the Sierra de El Abra region |
| Cartwright, R.A., Schwartz, R.S., Merry, A.L. and Howell, M.M. | Journal Article | 2016 | Strong selection is necessary for evolution of blindness in cave dwellers |
| Rossini, B.C., Oliveira, C.A.M., Melo, F.A.G., Bertaco, V.A., Astarloa, J.M.D., Rosso, J.J., Fauto, F. and Oliveira, C. | Journal Article | 2016 | Highlighting Astyanax Species Diversity through DNA Barcoding. |
| Lyon, A., Krutzler, A.J., Gross, J.B. and O'Quin, K.E. | Conference Paper | 2016 | Two to three genes control scleral ossification in blind Astyanax mexicanus cavefish |
| Elliott, W.R. | Book Section | 2016 | Cave exploration and mapping in the Sierra de El Abra Region |
| Tabin, C.J. | Book Section | 2016 | Introduction: The Emergence of the Mexican Cavefish as an Important Model System for Understanding Phenotypic Evolution |
| Jeffery, W.R. | Book Section | 2016 | Concluding remarks: The Astyanax community |
| O'Quin, K.E., Doshi, P., Lyon, A., Hoenemeyer, E., Yoshizawa, M. and Jeffery, W.R. | Conference Paper | 2016 | The evolution of scleral ossification in the Mexican Cavefish (Astyanax mexicanus) |
| Jeffery, W.R. | Conference Paper | 2016 | Homocystinuria in Cavefish: Molecular analysis of an Astyanax eye QTL reveals the role of cystathionine ß-synthase in eye degeneration |
| Jeffery, W.R. | Journal Article | 2016 | The comparative organismal approach in evolutionary developmental biology: Insights from ascidians and cavefish |
| Retaux, S., Alie, A., Blin, M., Devos, L., Elipot, Y. and Hinaux, H. | Book Section | 2016 | Neural development and evolution in Astyanax mexicanus: Comparing cavefish and surface fish brains |
| Gorički, S., Stanković, D., Snoj, A., Kuntner, M., Jeffery, W.R., Trontelj, P., Pavićević, M., Grizelj, Z., Năpăruş-Aljančič, M. and Aljančič, G. | Journal Article | 2017 | Environmental DNA in subterranean biology: range extension and taxonomic implications for Proteus |
| Cartwright, R.A., Schwartz, R.C., Merry, A.L. and Howell, M.M. | Journal Article | 2017 | The importance of selection in the evolution of blindness in cavefish |
| Retaux, S., Bibliowicz, J., Blin, M., Elipot, Y., Espinasa, L., Hinaux, H. and Tine, E. | Conference Paper | 2017 | Mechanisms underlying the evolution of olfactory capacities in blind cavefish |
| Mifsud. N. and Baker, N. | Journal Article | 2017 | Poissons mexicains: une fenêtre sur l’évolution. |
| Riddle, M., Finley, M., Marancik, D., Martineau, B. and Tabin, C. | Conference Paper | 2017 | What’s in your water? Pathogen landscape analysis of lab-raised Astyanax mexicanus; practical guidelines to increase biosecurity |
| Jaggard, J.B., Robinson, B.G., Stahl, B, Oh, I., Masek, P., Yoshizawa, M. and Keene, A. | Journal Article | 2017 | The lateral line confers evolutionarily derived sleep loss in the Mexican cavefish. |
| Krishnan, J., Seidel, C. and Rohner, N. | Conference Paper | 2017 | Regulatory evolution in cavefish metabolism |
| Krishnan, J. and Rohner, N. | Journal Article | 2017 | Cavefish and the basis for eye loss |
| Ornelas-Garcia, C.P., Powers, A., Berning, D., Rodiles, R., Barluenga, M. and Gross, J.B. | Conference Paper | 2017 | Transcriptome analyses and 3d geometric morphometrics characterization of lacustrine species pairs in Astyanax genus |
| Tuccinardi, M.J. | Journal Article | 2017 | Keeping Blind Cave Fishes (or not) |
| Stahl, B.A., Jaggard, J.B., Duboue, E.R. and Keene, A.C. | Conference Paper | 2017 | The evolution of neural circuitry regulating sleep and arousal in the blind mexican cavefish |
| Fernandes, V., Macaspac, C. and Yoshizawa, M. | Conference Paper | 2017 | How enhancement of sensory system is integrated to generate adaptive behavior |
| Hyacinthe, C., Aattia, J., Froc, C. and Retaux, S. | Conference Paper | 2017 | Evolution of acoustic communication in the blind cavefish Astyanax mexicanus |
| Yoshizawa, M., Valdez, C., Yew, J., Coyle, K. and Roberts, R. | Conference Paper | 2017 | Rapid evolution of the gut symbionts and asd-like behavior in the extreme cave environment |
| Collins, E., Rutkowski, J., Ornelas García, P., Rétaux, S., Rohner, N. and Espinasa, L. | Conference Paper | 2017 | Divergent evolutionary pathways for aggression and territoriality in Astyanax cave fish |
| Riddle, M.R.; Tabin, C.J | Conference Paper | 2017 | The eyeless Mexican cavefish Astyanax mexicanus as a model to investigate development and evolution of the gastrointestinal (GI) tract |
| Stahl, B.A. and Gross, J.B. | Journal Article | 2017 | A comparative transcriptomic analysis of development in two Astyanax cave populations |
| Aspiras, A.C., Riddle, M., Zueckert-Gaudenz, K., Peuß, R., Martineau, B., Box, A., Levy, M., Tabin, J.A., McGaugh, S., Borowsky, R., Tabin, C.J. and Rohner, N. | Conference Paper | 2017 | Insulin resistance in cavefish as an adaptation to a nutrient-poor environment |
| Espinasa, L., Bonaroti, N., Wong, J., Pottin, K., Queinnec, E. and Retaux, S. | Journal Article | 2017 | Contrasting feeding habits of post-larval and adult Astyanax cavefish |
| Kopp, J., Coppola, J. and Espinasa, L. | Conference Paper | 2017 | Phylogeographical convergence between two troglobitic organisms in the Sierra de El Abra |
| Alvarado, C.G., Gálvez Hernández, O., Garduño Sánchez, M. and Ornelas García, C.P. | Conference Paper | 2017 | Parallel evolution of body shape in Astyanax (Characidae) morphotypes |
| Powers, A.K., Davis, E.M., Kaplan, S.A. and Gross, J.B. | Journal Article | 2017 | Cranial asymmetry arises later in the life history of the blind Mexican cavefish, Astyanax mexicanus |
| Espinasa, L., Gibbon, D., Rutkowski, J., Collins, E. and Rohner, N. | Conference Paper | 2017 | Recuperation of vision in a blind cavefish population. |
| Bonaroti, N., Rutkowski, J., Rétaux, S., Collins, E., Ornelas García, P., Heintz, C. and Espinasa, L. | Conference Paper | 2017 | A year in the life of two populations of Astyanax mexicanus. |
| Poulson, T.L | Journal Article | 2017 | Book review: Wilkens and Strecker 2017 Evolution in the dark |
| Box, A.C., Peuß, R., Haug, J. and Rohner, N. | Conference Paper | 2017 | Immunology without antibodies: Studying fish hematopoietic tissue using intrinsic cell signals, image cytometry and advanced analysis methods |
| Powers, A.K., Davis, E.M., Kaplan, S.A. and Gross, J.B. | Conference Paper | 2017 | The evolution of cranial asymmetry across the life history of the blind mexican cavefish |
| Schmitter-Soto, J.J. | Journal Article | 2017 | A revision of Astyanax (Characiformes: Characidae) in Central and North America, with the description of nine new species |
| Delić, T., Trontelj, P., Rendoš, M. and Fišer, C. | Journal Article | 2017 | The importance of naming cryptic species and the conservation of endemic subterranean amphipods |
| Herman, A., Weagley, J., Jeffery, W.R., Borowsky, R., Brandvain, Y., Bilandžija, H., Espinasa, L., O'Quin, K., Ornelas-García, C., Gilbertson, E., Passow, C., Yoshizawa, M., Warren, W. and McGaugh, S.E. | Conference Paper | 2017 | Population genomics in Astyanax |
| Fumey, J., Hinaux, H., Noirot, C., Thermes, C., Retaux, S. and Casane, D. | Journal Article | 2017 | Evidence for Late Pleistocene origin of Astyanax mexicanus cavefish. |
| Peña-Herrejón, Guillermo A. Sanchez-Velazquez, Julieta Cruz-Hernández, Andrez Aguirre-Becerra, Humberto García-Trejo, Fernando | Conference Paper | 2017 | Breeding system for Astyanax mexicanus |
| Jaggard, J.B., Robinson, B.G., Stahl, B.A., Oh, I., Masek, P., Yoshizawa, M. and Keene, A.C. | Conference Paper | 2017 | The lateral line confers evolutionarily derived sleep loss in the mexican cavefish |
| Wilkens, H. and Strecker, U. | Book | 2017 | Evolution in the dark: Darwin's loss without selection |
| Lyon, A., Powers, A.K., Gross, J.B. and O'Quin, K.E. | Journal Article | 2017 | Two – three loci control scleral ossicle formation via epistasis in the cavefish Astyanax mexicanus |
| Torres-Paz, J., Alié, A., Devos, L., Prunier, L., Boulet, F., Blin, M., Elipot, Y. and Rétaux, S. | Conference Paper | 2017 | Developmental evolution of the prosencephalon in blind cavefish: Origins of natural variations in neuropeptidergic patterning and their behavioral consequences |
| Maldonado, E., García O.M. and Santacruz, A. | Conference Paper | 2017 | Evodevo and adaptations to perpetually dark environments: The left-right swimming preference in cave and surface morphs from Astyanax mexicanus |
| Gross, J.B. | Conference Paper | 2017 | Evolution and development of cranial asymmetry in Astyanax cavefish |
| Gore, A.V., Tomins, K., Iben, J., Castranova, D., Davis, A., Parkhurst, A., Soueidan, S., Jeffery, W.R. and Weinstein, B.M. | Conference Paper | 2017 | Role of DNA methylation in cavefish eye specific gene repression |
| Pierre, C., Froc, C., Callebert, J. and Rétaux, S. | Conference Paper | 2017 | Functional and behavioral consequences of the mutation in MAO in the blind cavefish Astyanax mexicanus |
| Riddle, M., Aspiras, A., Tabin, J., Damen, F., Martineu, B., Peavey, M. and Tabin, C. | Conference Paper | 2017 | It takes guts to live in a cave: Investigating the genetic basis of cave-specific gastrointestinal (gi) and metabolic adaptations |
| Di Tizio, L., Di Francesco, N., Pennelli, M. and Placentile, G. | Conference Paper | 2017 | Osservazioni in acquario sulla fenologia del caracide cieco delle grotte Astyanax jordani |
| Simon, N., Fujita, S., Porter, M. and Yoshizawa, M. | Conference Paper | 2017 | Extra-ocular opsin expression of Astyanax mexicanus cave and surface morphs |
| Gore, A.V., Tomins, K.A., Iben, J., Ma, L., Castranova, D., Davis, A.E., Parkhurst, A., Jeffery, W.R. and Weinstein, B.M. | Journal Article | 2017 | An epigenetic mechanism for cavefish eye degeneration |
| Tabin, J., Rohner, N., Haro, A., Kowalko, J., Martineau, B., Borowsky, R. and Tabin, C. | Conference Paper | 2017 | The evolution of temperature preference in the mexican cave fish Astyanax mexicanus |
| Kaplan, S.A., Powers, A.K. and Gross, J.B. | Conference Paper | 2017 | Understanding the Origin of Cranial Bone Fragmentation: Live-fluorescent Imaging Across Astyanax mexicanus Development |
| Simon, V., Elleboode, R., Mahé,K. Legendre, L., Ornelas‑Garcia, P., Espinasa, L. and Rétaux, S. | Journal Article | 2017 | Comparing growth in surface and cave morphs of the species Astyanax mexicanus: insights from scales |
| Jeffery, W.R., Ma, L., Tomins, K., Castranova, D., Weinstein, B.M. and Gore, A. | Conference Paper | 2017 | Analysis of the eye QTL gene CBSA reveals the role of cranial vasculature in regression of the cavefish visual system |
| Hinaux, H., Recher, G., Alie, A., Legendre, L., Blin, M. and Retaux, S. | Journal Article | 2017 | Lens apoptosis in the Astyanax blind cavefish is not triggered by its small size or defects in morphogenesis |
| Ma, L., Strickler, A.G., Parkhurst, A., Yoshizawa, M., Shi, J. and Jeffery, W.R. | Journal Article | 2018 | Maternal genetic effects in Astyanax cavefish development |
| Alie, A., Devos, L., Torres-Paz, J., Prunier, L., Boulet, F., Blin, M., Elipot, Y. and Retaux, S. | Journal Article | 2018 | Developmental evolution of the forebrain in cavefish, from natural variations in neuropeptides to behavior |
| Tang, J.L.Y., Guo, Y., Stockdale, W.T., Rana, K., Killen, A.C., Mommersteeg, M.T.M. and Yamamoto, Y. | Journal Article | 2018 | The developmental origin of heart size and shape differences in Astyanax mexicanus populations |
| Atukorallaya, D.S.A. and Ratnayake, R.K. | Journal Article | 2018 | Shaping the craniofacial skeleton of Mexican cavefish (Astyanax mexicanus); Role of Osteoblast and Osteoclast. |
| Xiong, S., Krishnan, J., Peuß, F. and Rohner, N. | Journal Article | 2018 | Early adipogenesis contributes to excess fat accumulation in cave populations of Astyanax mexicanus |
| Carlson, B.M. and Gross, J.B. | Journal Article | 2018 | Characterization and comparison of activity profiles exhibited by the cave and surface morphotypes of the blind Mexican tetra, Astyanax mexicanus |
| Ren, X.Y., Hamilton, N., Müller, F. and Yamamoto, Y. | Journal Article | 2018 | Cellular rearrangement of the prechordal plate contributes to eye degeneration in the cavefish |
| Stockdale, W.T., Lemieux, M.E., Killen, A.C., Zhao, J., Hu, J., Riepsaame, J. Hamilton, N., Kudoh, T., Riley, P.R., van Aerle, R., Yamamoto, Y. and Mommersteeg, M.T.M. | Journal Article | 2018 | Heart regeneration in the Mexican cavefish |
| Espinasa, L. | Book Section | 2018 | The Guerrero fish population: Astyanax aeneus as a comparative cavefish model |
| Carlson, B.M., Klingler, I.B., Meyer, B.J. and Gross, J.B. | Journal Article | 2018 | Genetic analysis reveals candidate genes for activity QTL in the blind Mexican tetra, Astyanax mexicanus |
| Jeffery, W.R. | Conference Paper | 2018 | Maternal genetic effects in Astyanax cavefish development |
| Sears, C.R. and Gross, J.B. | Conference Paper | 2018 | The RNA Architecture of Life in the Dark: A Transcriptomic Assessment of Varying Photic Conditions in the Blind Mexican Cavefish, Astyanax mexicanus |
| Elliott, W.R. | Book | 2018 | The Astyanax Caves of Mexico: Cavefishes of San Luís Potosí, Tamaulipas, and Guerrero |
| Espinasa, L., Legendre, L., Fumey, J., Blin, M., Rétaux, S. and Espinasa, M. | Journal Article | 2018 | A new cave locality for Astyanax cavefish in Sierra de El Abra, Mexico |
| Davis, V.A., Holbrook, R.I. and Burt de Perera, T. | Journal Article | 2018 | The influence of locomotory style on three-dimensional spatial learning |
| Torres-Dowdall, J., Karagic, N., Plath, M. and Riesch, R. | Journal Article | 2018 | Evolution in caves: selection from darkness causes spinal deformities in teleost fishes |
| Devitsina, G.V. and Golovkina, T.V. | Journal Article | 2018 | Structural Organization of the Taste Apparatus in Characins (Characidae, Teleostei |
| Mirande, J.M. | Journal Article | 2018 | Morphology, molecules and the phylogeny of Characidae (Teleostei, Characiformes) |
| Klaassen, H., Wang, Y., Adamski, K., Rohner, N. and Kowalko, J.E. | Journal Article | 2018 | CRISPR mutagenesis confirms the role of oca2 in melanin pigmentation in Astyanax mexicanus |
| Koch, L. | Journal Article | 2018 | All eyes on rapid adaptive evolution |
| Powers, A.K., Boggs, T.E. and Gross, J.B. | Journal Article | 2018 | Canal neuromast position prefigures developmental patterning of the suborbital bone series in Astyanax cave- and surface-dwelling fish |
| Borowsky, R. | Journal Article | 2018 | Primer. Cavefishes |
| Rohner, N. | Book Section | 2018 | “Out of the dark” Cavefish are entering biomedical research |
| Tabin, J.A., Aspiras, A., Martineau, B., Riddle, M., Kowalko, J., Borowsky, R., Rohner, N. and Tabin, C.J. | Journal Article | 2018 | Temperature preference of cave and surface populations of Astyanax mexicanus |
| Riddle, M.R., Aspiras, A.C., Gaudenz, K., Peuß, R., Sung, J.Y., Martineau, B., Peavey, M., Box, A.C., Tabin, J.A., McGaugh, S., Borowsky, R., Tabin, C.J. and Rohner, N. | Journal Article | 2018 | Insulin resistance in cavefish as an adaptation to a nutrient-limited environment |
| Borowsky, R., Luk, A., He, X.J. and Kim, R.S. | Journal Article | 2018 | Unique sperm haplotypes are associated with phenotypically different sperm subpopulations in Astyanax fish |
| Ornelas-García, P., Pajares, S., Sosa-Jiménez, V.M., Rétaux, S. and Miranda-Gamboa, R.A. | Journal Article | 2018 | Microbiome differences between river-dwelling and caveadapted populations of the fish Astyanax mexicanus (De Filippi, 1853) |
| Pagano, C., Siauciunaite, R., Idda, M.L., Ruggiero, G., Ceinos, R.M., Pagano, M., Frigato, E., Bertolucci, C., Foulkes, N.S. and Vallone, D. | Journal Article | 2018 | Evolution shapes the responsiveness of the D-box enhancer element to light and reactive oxygen species in vertebrates |
| Yoshizawa, M., Settle, A., Hermosura, M.C., Tuttle, L.J., Cetraro, N., Passow, C.N. and McGaugh, S.E. | Journal Article | 2018 | The evolution of a series of behavioral traits is associated with autism-risk genes in cavefish |
| Fernandes, V.F.L., Macaspac, C., Lu, L. and Yoshizawa, M. | Journal Article | 2018 | Evolution of the developmental plasticity and a coupling between left mechanosensory neuromasts and an adaptive foraging behavior |
| Rohner, N. | Journal Article | 2018 | Cavefish as an evolutionary mutant model system for human disease |
| Fumey, J., Hinaux, H., Noirot, C., Thermes, C., Rétaux, S. and Casane, D. | Journal Article | 2018 | Evidence for late Pleistocene origin of Astyanax mexicanus cavefish |
| Riddle, M.R., Boesmans, W., Caballero, O, Kazwiny, Y. and Tabin,C.J. | Journal Article | 2018 | Morphogenesis and motility of the Astyanax mexicanus gastrointestinal tract |
| Atukorala, A.D.S. and Franz-Odendaal, T.A. | Journal Article | 2018 | Genetic linkage between altered tooth and eye development in lens-ablated Astyanax mexicanus |
| Garita-Alvarado, C.A., Barluenga, M. and Ornelas-García, C.P. | Journal Article | 2018 | Parallel evolution of morphs of Astyanax species (Teleostei: Characidae) in México and Central America |
| Frøland Steindal, I.V., Beale, A.D., Yamamoto, Y. and Whitmore, D. | Journal Article | 2018 | Development of the Astyanax mexicanus circadian clock and non-visual light responses |
| Gross, J.B., Weagley, J., Stahl, B.A., Ma, L., Espinasa, L. and McGaugh, S.E. | Journal Article | 2018 | A local duplication of the Melanocortin receptor 1 locus in Astyanax |
| Porter, M.L. and Sumner‐Rooney, L. | Conference Paper | 2018 | Evolution in the dark: Unifying our understanding of eye loss |
| Pazza, R., Dergam, J.A. and Kavalco, K.F. | Journal Article | 2018 | Trends in karyotype evolution in Astyanax (Teleostei, Characiformes, Characidae): Insights from molecular data. |
| Shiriagin, V. and Korsching, S.I. | Journal Article | 2018 | Massive expansion of bitter taste receptors in blind cavefish, Astyanax mexicanus |
| Espinasa, L., Hoese, G., Toulkeridis, T. and Toomey, R. | Journal Article | 2018 | Corroboration that the Mc1rGly/Ser mutation correlates with the phenotypic expression of pigmentation in Astroblepus |
| Retaux, S. | Journal Article | 2018 | The healthy diabetic cavefish conundrum |
| Riddle, M., Martineau, B., Peavey, M. and Tabin, C. | Journal Article | 2018 | Raising the Mexican tetra Astyanax mexicanus for analysis of post-larval phenotypes and whole-mount immunohistochemistry. |
| Bilandzija, H., Abraham, L., Ma, L., Renner, K.J., & Jeffery, W.R. | Journal Article | 2018 | Behavioural changes controlled by catecholaminergic systems explain recurrent loss of pigmentation in cavefish |
| Blin, M., Tine, E., Meister, L., Elipot, Y., Bibliowicz, J., Espinasa, L. and Rétaux, S. | Journal Article | 2018 | Developmental evolution and developmental plasticity of the olfactory epithelium and olfactory skills in Mexican cavefish |
| Espinasa, L., Robinson, J. and Espinasa, M. | Journal Article | 2018 | Mc1r gene in Astroblepus pholeter and Astyanax mexicanus: Convergent regressive evolution of pigmentation across cavefish species |
| Lloyd, E., Olive, C., Stahl, B.A., Jaggarda, J.B., Amaral, P., Dubouéa, E.R and Keene, A.C. | Journal Article | 2018 | Evolutionary shift towards lateral line dependent prey capture behavior in the blind Mexican cavefish |
| Helena, B., Lindsey, A., Li, M., Kenneth, J.R. and William, R.J. | Journal Article | 2018 | Behavioural changes controlled by catecholaminergic systems explain recurrent loss of pigmentation in cavefish |
| Ahmad, H., Azizan, T.R.P.T., Noor, M.H.M., Hassim, H.A. and Daud, H.M. | Journal Article | 2018 | Development of a Y-maze task for studying cognitive function using teleost zebrafish (Danio rerio) and cavefish (Astyanax mexicanus) |
| Herman, A., Brandvain, Y., Weagley, J., Jeffery, W.R., Keene, A.C., Kono, T.J., Bilandžija, H., Borowsky, R., Espinasa, L., O'Quin, K., Ornelas-García, C.P., Yoshizawa, M., Carlson, B, Maldonado E, Gross JB, Cartwright RA, Rohner N, Warren WC, McGaugh SE. | Journal Article | 2018 | The role of gene flow in rapid and repeated evolution of cave‐related traits in Mexican tetra, Astyanax mexicanus |
| Jaggard, J.B., Stahl, B.A., Lloyd, E., Prober, D.A., Duboue, E.R. and Keene, A.C. | Journal Article | 2018 | Hypocretin underlies the evolution of sleep loss in the Mexican cavefish |
| Kling, J. | Journal Article | 2018 | Insulin resistance grows in the dark |
| Keene, A.C.and Duboue, E.R. | Journal Article | 2018 | The origins and evolution of sleep |
| Kopp, J., Avasthi, S. and Espinasa, L. | Journal Article | 2018 | Phylogeographical convergence between Astyanax cavefish and mysid shrimps in the Sierra de El Abra, Mexico |
| Torres-Paz,J., Hyacinthe, C., Pierre, C., Retaux, S. | Journal Article | 2018 | Towards an integrated approach to understand Mexican cavefish evolution |
| Chin, J.S.R., Gassant, C.E., Amaral, P.M., Lloyd, E., Stahl, B.A., Jaggard, J.B., Keene, A.C. and Duboue, E.R. | Journal Article | 2018 | Convergence on reduced stress behavior in the Mexican blind cavefish |
| Zhao, H., Di Mauro, G., Lungu-Mitea, S., Negrini, P., Guarino, A.M., Frigato, E., Braunbeck, T., Ma, H., Lamparter, T., Vallone, D., Bertolucci, C. and Foulkes, N.S. | Journal Article | 2018 | Modulation of DNA repair systems in blind cavefish during evolution in constant darkness |
| Kasumyana, A.O. and Marusova, E.A. | Journal Article | 2018 | Odor stimulation and relation to taste stimuli in the blind cave fish Astyanax fasciatus |
| Ojha, A. and Watve, M. | Journal Article | 2018 | Blind fish: An eye opener |
| Bussotti, S., Di Franco, A., Bianchi, C.N., Chevaldonné, P., Egea, L., Fanelli, E., Lejeusne, C., Musco, L., Navarro-Barranco, C., Pey, A. and Planes, S. | Journal Article | 2018 | Fish mitigate trophic depletion in marine cave ecosystems |
| Ornelas-García, P,. Pajares, S., Sosa-Jiménez, V.M., Rétaux, S. and Miranda-Gamboa, R.A. | Journal Article | 2018 | Microbiome differences between riverdwelling and cave-adapted populations of the fish Astyanax mexicanus (De Filippi, 1853) |
| Ceinos, R., Frigato, E., Pagano, C., Fröhlich, N., Negrini, P., Cavallari, N., Vallone, D., Fuselli, S., Bertolucci, C. and Foulkes, N. | Journal Article | 2018 | Mutations in blind cavefish target the light-regulated circadian clock gene, period 2 |
| Kasumyan, A.O. and Marusov, E.A. | Journal Article | 2018 | [Odor stimulation and relation to taste stimuli in the blind cave fish Astyanax fasciatus] |
| Stahl, B.A., Sears, C.R., Ma, L., Perkins, M., & Gross, J.B. | Book Section | 2018 | Pmela and Tyrp1b contribute to melanophore variation in Mexican cavefish |
| Gore, A.V., Tomins K.A., Iben, J., Ma, L., Castranova, D., Davis, A.E., Parkhurst, A., Jeffery, W.R. and Weinstein, B.M. | Journal Article | 2018 | An epigenetic mechanism for cavefish eye degeneration |
| Gore, A., Jeffery, W., Retaux, S. and Rohner, N. | Book | 2018 | Cavefish development |
| Sovrano, V.A., Potrich, D., Foà, A. and Bertolucci, C. | Journal Article | 2018 | Extra-visual systems in the spatial reorientation of cavefish |
| Stern, D.B and Crandall, K.A. | Journal Article | 2018 | The evolution of gene expression underlying vision loss in cave animals |
| Powers, A.K., Kaplan, S.A., Boggs, T.E. and Gross, J. | Journal Article | 2018 | Facial bone fragmentation in blind cavefish arises through two unusual ossification processes |
| Gore, A.V., Rohner, N., Rétaux, S. and Jeffery, W.R. | Journal Article | 2018 | Seeing a bright future for a blind fish |
| Riddle, M.R., Aspiras, A., Damen, F., Hutchinson, J.N., Chinnapen, D. and Tabin, C.J. | Journal Article | 2019 | Genetic architecture underlying changes in carotenoid accumulation during the evolution of the Blind Mexican cavefish, Astyanax mexicanus |
| Borowsky, R., Luk, A. and Kim, R.S. | Journal Article | 2019 | Sperm swimming behaviors are correlated with sperm haploid genetic variability in the Mexican tetra, Astyanax mexicanus |
| Jeffery, W.R. | Book Section | 2019 | Astyanax mexicanus: A vertebrate model for evolution, adaptation, and development in caves |
| Chin, J.S.R., Loomis, C.L., Phan, T.A.N., Albert, L.T., Chang, L.P., Keene, A.C. and Duboué, E.R. | Conference Paper | 2019 | Diminished stress responses in the Mexican blind cavefish |
| Potts, H.G., Stockdale, W.T., Choudhury, R.P. and Mommersteeg, M.T.M. | Conference Paper | 2019 | Investigating the role of CD11b/ITGAM, a leukocyte adhesion protein, in cardiac regeneration |
| Devos, L., Klee, F., Edouard, J., Simon, V., Legendre, L., Khallouki, N.E., Barbachou, S., Sohm, F. and Rétaux, S. | Journal Article | 2019 | Morphogenetic and patterning defects explain the coloboma phenotype of the eye in the Mexican cavefish |
| Sears, C.R. | Thesis | 2019 | Transcriptomic analysis of the effect of dark-rearing on Astyanax mexicanus |
| Keene, A.C. | Conference Paper | 2019 | Genetic and evolutionary regulation of sleep-feeding interactions |
| Jaggard, J.B., Lloyd, E., Lopatto, A., Duboue, E.R. and Keene, A.C. | Journal Article | 2019 | Automated measurements of sleep and locomotor activity in Mexican cavefish. |
| Gil-Galvez, A., de la Calle Mustienes, E., Neto, A.., Bilandžija, H., Jeffery, W.R., Gómez-Skarmeta, J.L. | Conference Paper | 2019 | The role of Cis-Regulatory elements in morphological adaptation to cave environment in Astyanax mexicanus |
| Kling, J. | Journal Article | 2019 | To wild depths for new models |
| Hu, Z., Lemieux, M. and Mommersteeg, M. | Conference Paper | 2019 | Differences in extracellular matrix deposition during heart regeneration and scarring in Astyanax mexicanus |
| Fernandes, C.S., Batalha, M.A., Bichuette, M.E. | Journal Article | 2019 | Dark diversity in the dark: a new approach to subterranean conservation |
| Reyes Corral, W.D. and Aguirre, W.E. | Journal Article | 2019 | Effects of temperature and water turbulence on vertebral number and body shape in Astyanax mexicanus (Teleostei: Characidae) |
| Stockdale, W.T., Tang, J.L.Y., Hu, Z., Potts, H.G., Lemieux, M.E., Yamamoto, Y. and Mommersteeg, M.T.M. | Conference Paper | 2019 | Heart development and regeneration in Astyanax mexicanus |
| Borowsky, R. | Conference Paper | 2019 | Gene flow from surface to cave populations of Astyanax mexicanus is ongoing |
| Powers, A.K., Boggs, T.E., Kaplan, S.A., Davis, E.M. and Gross, J.B. | Conference Paper | 2019 | Sensory-skeletal integration and cranial asymmetry in cavefish |
| Leclercq, J., Torres-Paz, J. and Rétaux, S. | Conference Paper | 2019 | Impact of maternal factors during evolution of gastrulation and forebrain morphogenesis in Astyanax mexicanus |
| Berning, D., Adams, H., Luc, H. and Gross, J.B. | Journal Article | 2019 | In-frame indel mutations in the genome of the blind Mexican cavefish, Astyanax mexicanus |
| Policarpo, M., Fumey, J., Lafargeas, P., Legendre, L., Naquin, D., Thermes, C., Møller, P.R., Bernatchez, L., García-Machado, E., Rétaux, S. and Casane, D. | Conference Paper | 2019 | Molecular decay of light-processing genes in cavefishes: assessing the effects of time, genome architecture and gene flow |
| Gallman, K., Rivera, D. and Soares, D. | Journal Article | 2019 | Evolutionary increases in catecholamine signaling may underlie the emergence of adaptive traits and behaviors in the blind cavefish, Astyanax mexicanus |
| Warren, W.C., Koren, S., Meyer, A. and Rohner, N. | Conference Paper | 2019 | The next generation of Asytanax mexicanus genome references |
| Hyacinthe, C., Attia, J. and Retaux, S. | Conference Paper | 2019 | Evolution of acoustic communication in the blind cavefish Astyanax mexicanus |
| Legendre, L., Pere, S. and Rétaux, S. | Conference Paper | 2019 | Water quality of rivers and caves inhabited by Astyanax mexicanus surface fish and cavefish |
| Stahl, B.A., Peuß, R., McDole, B., Kenzior, A., Jaggard, J.B., Gaudenz, K., Krishnan, J., McGaugh, S.E., Duboue, E.R., Keene, A.C. and Rohner, N. | Journal Article | 2019 | Stable transgenesis in Astyanax mexicanus using the Tol2 transposase system |
| Agnès, F., Devos, L. and Rétaux, S. | Conference Paper | 2019 | The embryonic eyefield of the cavefish Astyanax mexicanus |
| McGaugh, S.E., Passow, C., Jaggard, J.B., Stahl, B. and Keene, A. | Conference Paper | 2019 | Sleep deprivation evokes unique expression responses across independently evolved cavefish |
| Luc, H., Sears, C., Raczka, A. and Gross, J.B. | Journal Article | 2019 | Wholemount in situ hybridization for Astyanax embryos |
| Rétaux, S., Bibliowicz, Y., Blin, M., Elipot, Y., Espinasa, L., Hinaux, H. and Tine, E. | Conference Paper | 2019 | Olfactory capacities in blind cavefish |
| Legendre, L., Guillet, B., Leguay, E., Meunier, E., Keck, N. and Sohm, F. | Conference Paper | 2019 | RESAMA : French network for aquatic model animal health Monitoring. Focus on Astyanax mexicanus |
| Fernández, A.A., Evans, E.I. and Baumann, D.P. | Conference Paper | 2019 | The importance of health monitoring in Astyanax mexicanus |
| Pennisi, E. | Web Page | 2019 | A lopsided face helps this eyeless cave fish navigate |
| van der Laan, R. | Book | 2019 | Freshwater fish list. An alphabetic scientific name list of the world's freshwater fishes and an overview of the scientific names used in the aquarium literature |
| Culver, D.C. and Pipan, T. | Book | 2019 | The biology of caves and other subterranean habitats. Second edition. |
| Wilkens, H | Conference Paper | 2019 | The primacy of the genotype |
| Van der Weele, C.M. and Jeffery, W.R. | Journal Article | 2019 | Cavefish increase red blood cell development and reprogram metabolism as adaptations to environmental hypoxia. |
| Worsham, M., Fang, J. and Yoshizawa, M. | Conference Paper | 2019 | Epigenetic regulation of phenotype of Mexican tetras |
| Krishnan, J. and Rohner, N. | Journal Article | 2019 | Sweet fish: Fish models for the study of hyperglycemia and diabetes |
| O’Gorman, M., Lewis, P., Keene, A., Kowalko, J. and Duboué, E. | Conference Paper | 2019 | Adapting zebrafish rearing techniques for Astyanax mexicanus |
| Jazwinska, A. and Blanchoud, S. | Journal Article | 2019 | Towards deciphering variations of heart regeneration in fish |
| Pastana, M.N.L., Bockmann, F.A. and Datovo, A. | Journal Article | 2019 | The cephalic lateral-line system of Characiformes (Teleostei: Ostariophysi): anatomy and phylogenetic implications |
| Blin, M., Fumey, J., Père, S., Pierre, C., Torres-Paz, J., Simon, V., Espinasa, L. Legendre, L., Casane, D. and Rétaux, S. | Conference Paper | 2019 | Olfactory behavior of different wild Astyanax mexicanus populations |
| Fernandes, V.F.L., Hernandez, H., Doeden, N. and Yoshizawa, M. | Conference Paper | 2019 | Evolution of sensory-behavior laterality in Astyanax mexicanus |
| Devos, L., Edouard, J., Simon, V., Agnès, F., Sohm, F. and Rétaux, S. | Conference Paper | 2019 | Eye morphogenesis in the blind Mexican cavefish |
| Ma, L. and Jeffery, W.R. | Conference Paper | 2019 | Global analysis of maternal transcripts in Astyanax cavefish eggs |
| Loomis, C., Peuß, R., Jaggard, J., Wang, Y., McKinney, S., Raftopoulos, S., Raftopoulos, A., Whu, D., Green, M., McGaugh, S.E., Rohner, N., Keene, A.C., Duboue, E.R. | Journal Article | 2019 | An adult brain atlas reveals broad neuroanatomical changes in independently evolved populations of Mexican cavefish |
| Loomis, C., Peuss, R., Jaggard, J.B., Wang, Y., McKinney, S.A., Raftopoulos, S.C., … Duboue, E.R. | Journal Article | 2019 | An adult brain atlas reveals broad neuroanatomical changes in independently evolved populations of Mexican cavefish |
| Lloyd, E., Olive, C., Stahl, B.A., Jaggard, J.B., Amaral, P., Duboué, E.R. and Keene, A.C. | Conference Paper | 2019 | Evolutionary shift towards lateral line dependent prey-capture in the Mexican Cavefish |
| Boggs, T.E. and Gross, J.B. | Conference Paper | 2019 | Exploring the role of blood physiology in cave adaptation |
| Atukorallaya, D., Bhatia, V. and Ratnayake, R. | Conference Paper | 2019 | Tooth and jaw development in health and disease; Insight form Mexican tetra (Astyanax mexicanus) |
| Jaggard, J., Lloyd, E., Loomis, C., Cook, C., Vega, P., McGaugh, S., Rohner, N., Bohrner, J., Duboue, E. and Keene, A.C. | Conference Paper | 2019 | A cavefish brain atlas reveals functional and anatomical evolution of morphology and neural activity with convergence of sleep and feeding circuits |
| Hyacinthe, C., Attia, J. and Retaux, S. | Journal Article | 2019 | Evolution of acoustic communication in blind cavefish |
| Barash, D.P. | Journal Article | 2019 | Review of: Wilkens and Strecker (2017) Evolution in the Dark: Darwin’s Loss Without Selection |
| Duboue, E. | Conference Paper | 2019 | Evolution of stress responses in Mexican cavefish |
| Mogdans, J. | Journal Article | 2019 | Sensory ecology of the fish lateral-line system: Morphological and physiological adaptations for the perception of hydrodynamic stimuli |
| McDole, B., Jaggard, J., Duboue, E. and Keene, A.C. | Conference Paper | 2019 | Multiphoton ablation of HCRT neurons increases sleep in the Molino cavefish population |
| Kibele, C.S., Montgomery, J.C. and Radford, C.A. | Journal Article | 2019 | The use of evoked potentials to determine sensory sub‑modality contributions to acoustic and hydrodynamic sensing |
| Garduño-Sánchez, M.A.A. and Ornelas-García, C.P. | Conference Paper | 2019 | Molecular evolution of rhodopsin gene in Astyanax mexicanus (De Filippi, 1853) |
| Loomis, C., Peuss, R., Green, M., McGaugh, S.E., Rohner, N., Keene, A.C. and Duboue, E.R. | Conference Paper | 2019 | Generation of a brain atlas for adult Astyanax reveal differences in distinct neuroanatomical sub-nuclei associated with cave-specific behaviors |
| Riddle, M., Damen, F., Aspiras, A., Tabin, J., McGaugh, S. and Tabin, C. | Conference Paper | 2019 | Investigating the genetic and developmental basis of gut length plasticity in A. mexicanus |
| Simon, V., Hyacinthe, C. and Retaux, S. | Journal Article | 2019 | Breeding behavior in the blind Mexican cavefish and its river‐dwelling conspecific |
| Peuß, R., Box, A.C., Wang, Y., Chen, S., Krishnan, J., Tsuchiya, D., Peak, A., Perera, A., Slaughter, B. and Rohner, N. | Conference Paper | 2019 | Cave adaptation shifts immune cell composition compensating increased inflammatory responses in Astyanax mexicanus |
| Keene, A.C. and Appelbaum, L. | Journal Article | 2019 | Sleep in fish models |
| Maximino, C., do Carmo Silva, R.X., dos Santos Campos, K., de Oliveira, J.S., Rocha, S.P., Pyterson, M.P., dos Santos Souza, D.P., Feitosa, L.M., Ikeda, S.R., Pimentel, A.F.N., Pâmila N. F. | Journal Article | 2019 | Sensory ecology of ostariophysan alarm substances |
| Paz, A., McDole, B., Duboue, E. and Keene, A.C. | Conference Paper | 2019 | Analysis of startle responses in A. mexicanus reveals possible effects of diminished predation |
| Riddle, M.R. and Tabin, C.J. | Journal Article | 2019 | Little fish, big questions: A collection of modern techniques for Mexican tetra research |
| Torres-Paz, J., Leclercq. J. and Rétaux, S. | Conference Paper | 2019 | Evolution of gastrulation and forebrain morphogenesis in Astyanax mexicanus |
| McNeill-Jewer, C.A., Reinhardt, E.G., Collins, S., Kovacs, S., Chan, W.M., Devos and LeMaillot, F.C. | Journal Article | 2019 | The effect of seasonal rainfall on nutrient input and biological productivity in the Yax Chen cave system (Ox Bel Ha), Mexico, and implications for μXRF core studies of paleohydrology |
| Kowalko, J.E. | Conference Paper | 2019 | Elucidating the genetic basis of trait evolution in the cavefish |
| van der Weele, C.M. and Jeffery, W.R. | Conference Paper | 2019 | Cavefish embryos develop more red blood cells as an adaptation to hypoxia |
| McGaugh, S.E., Passow, C.N., Jaggard, J.B., Stahl, B.A. and Keene, A.C. | Journal Article | 2019 | Unique transcriptional signatures of sleep loss across independently evolved cavefish populations |
| Mcgaugh, S.E., Weaver, S., Gilbertson, E.N., Garrett, B., Rudeen, M.I., Grieb, S., Roberts, J., Donny, A., Marchetto, P. and Gluesenkamp, A,G. | Journal Article | 2019 | Evidence for rapid phenotypic and behavioural shifts in a recently established cavefish population |
| Emam, A., Yoffe, M., Cardona, H. and Soares, D. | Journal Article | 2019 | Retinal morphology in Astyanax mexicanus during degeneration |
| Krishnan, J. and Rohner, N. | Conference Paper | 2019 | Regulatory evolution of metabolic adaptations in cavefish |
| Bilandžija, H., Hollifield, B., Steck, M., Meng, G., Ng, M., Bedek, J., Ćetković, H., Porter, M., Renner, K.J. ands Jeffery, W.R. | Conference Paper | 2019 | Phenotypic plasticity and genetic assimilation in the evolution of cave-adapted traits in Astyanax mexicanus |
| Bilandžija, H., Hollifield, B., Steck, M., Meng, G., Ng, M., Koch, A.D., Gračan, R., Ćetković, H., Porter, M.L., Renner, K.J. and Jeffery, W.R. | Journal Article | 2019 | Phenotypic plasticity as a mechanism of cave colonization and adaptation |
| Yoshizawa, M., Valdez, C., Balaan, C., Lactaoen, K., Kato, J., Ito, M., Yew, J. and Iwashita, M. | Conference Paper | 2019 | Reciprocal social Interaction was recovered under the treatment of ketogenic diet |
| Sifuentes-Romero I. and Kowalko, J.E. | Conference Paper | 2019 | Morphological variations in eye size: does rx3 play a role? |
| Coppola, J. and Espinasa, L. | Journal Article | 2019 | Cave Astyanax: Hunters or scavengers? Evidence from gut contents |
| Hernández, J., Garita-Alvarado, C.A. and Ornelas-García, C.P. | Conference Paper | 2019 | Parallel trophic morphology in cave and surface fish Astyanax mexicanus (De Filippi 1853) |
| Stahl, B.A., Peuß, R., McDole, B., Kenzior, A., Jaggard, J.B., Gaudenz, K., Krishnan, J., McGaugh, S.E., Duboue, E.R., Keene, A.C and Rohner, N. | Journal Article | 2019 | Stable transgenesis in Astyanax mexicanus using the Tol2 transposase system |
| Phelps, A. and Gross, J. | Conference Paper | 2019 | Examination of pigmentation differences in cave and surface Astyanax mexicanus using in situ hybridization |
| Imarazene, B., Herpin, A., Feron, R., Postlethwait, J.H., Schartl, M., Retaux, S. and Guiguen, Y. | Conference Paper | 2019 | Micro-evolution of sex determination mechanisms and sex determining genes in the cavefish, Astyanax mexicanus |
| Espinasa, L., Retaux, S., Yoshisawa, M., Heintz, C. and Balogh-Robinson, R. | Conference Paper | 2019 | Vibration Attraction Behavior expression is prevalent among caves, but variable within caves |
| Jeffery, W.R., Ma, L., Shi, J., Ng, M. and Gore,A. | Conference Paper | 2019 | Evolution of left-right axis asymmetry in Astyanax cavefish |
| Mogdans, J. | Journal Article | 2019 | Sensory ecology of the fish lateral line system: morphological and physiological adaptations for the perception of hydrodynamic stimuli |
| McGaugh, S.E., Weaver, S., Gilbertson, E.N., Garrett, B., Rudeen, M.L., Grieb, S., Roberts, J., Donny, A., Marchetto, P. and Gluesenkamp, A.G. | Journal Article | 2019 | Evidence for rapid phenotypic and behavioral change in a recently established cavefish population |
| Piscor, D., Pozzobon, A.P.B., Fernandes, C.A., Centofante, L, and Parise-Maltempi, P.P. | Journal Article | 2019 | Molecular clock as insight to estimate the evolutionary history and times of divergence for 10 nominal Astyanax species (Characiformes, Characidae): An evolutionary approach in species with 2n = 36, 46, 48, and 50 chromosomes |
| Yoffe, M., Patel, K., Palia, E., Kolawole, S., Streets, A., Haspel, G. and Soares, D. | Journal Article | 2019 | Hunting strategies and the lateral line of Astyanax mexicanus larva |
| Palluzzi, T., Rowland, R. and Gross, J. | Conference Paper | 2019 | The effect of neuromast position on bone fragmentation in the third suborbital bone of Astyanax mexicanus |
| Ornelas-García, C.P., Pajares, S., Sosa-Jiménez, V.M., Rétaux, S. and Gamboa-Miranda, R. | Conference Paper | 2019 | Microbiome characterization of cave-adapted and surface populations of the fish Astyanax mexicanus (De Filippi, 1853) |
| Torres-Paz, J., Leclercq, J. and Rétaux, S. | Journal Article | 2019 | Maternally regulated gastrulation as a source of variation contributing to cavefish forebrain evolution |
| Stahl, B.A., Peuß, R., McDole, B., Kenzior, A., Jaggard, J.B., Gaudenz, K., Krishnan, J., McGaugh, S.E., Duboue, E.R., Keene, A.C. and Rohner, N. | Conference Paper | 2019 | Stable transgenesis in Astyanax mexicanus using the Tol2 transposase system |
| Powers, A.K., Garita-Alvarado, C., Rodiles, R., Berning, D.J., Gross, J.B. and Ornelas-García, C.P. | Conference Paper | 2019 | Cranial plasticity in lake-dwelling Astyanax morphotypes corresponds to feeding preference |
| Stahl, B.A., Jaggard, J.B., Chin, J.S., Kowalko, J.E., Keene, A.C. and Duboué, E.R. | Journal Article | 2019 | Manipulation of gene function in Mexican cavefish |
| Worsham, M., Fernandes, V.F.L., Settle, A., Balaan, C., Lactaoen, K., Tuttle, L.J., Iwashita, M. and Yoshizawa, M. | Journal Article | 2019 | Behavioral tracking and neuromast imaging of Mexican cavefish. |
| Gluesenkamp, A. and McGaugh, S. | Conference Paper | 2019 | The Texas Tetra Project |
| Atukorala, A.D.S., Bhatia, V. and Ratnayake, R. | Journal Article | 2019 | Craniofacial skeleton of Mexican tetra (Astyanax mexicanus): As a bone disease model |
| Kindl, G.H. and O’Quin, K.E. | Journal Article | 2019 | On intra- and inter-specific variation in teleost scleral ossification |
| Peuß, R., Zakibe, Z., Krishnan, J., Merryman, M.S., Baumann, D.P. and Rohner, N. | Journal Article | 2019 | Gamete collection and In vitro fertilization of Astyanax mexicanus |
| Betancur-R.R., Arcila, D., Vari, R.P. et al. | Journal Article | 2019 | Phylogenomic incongruence, hypothesis testing, and taxonomic sampling: the monophyly of characiform fishes |
| Pierre, C., Torres-Paz, J., Froc, C., Callebert, J. and Rétaux, S. | Conference Paper | 2019 | Functional and behavioral consequences of the mutation in monoamine oxidase (MAO) in the blind cavefish Astyanax mexicanus |
| Stockdale, W.T., Deal, O., Heintz, C., Rohner, N., and Mommersteeg, M.T.M. | Conference Paper | 2019 | Glucose metabolism during heart regeneration in the Astyanax mexicanus |
| Shimobayashi, M., Shetty, S., Frei, I.C., Wölnerhanssen, B.K., Weissenberger, D., Dietz, N., Thomas, A., Ritz, D., Meyer-Gerspach, A.C., Maier, T., Hay, N., Peterli, R., Rohner, N. | Journal Article | 2019 | Diet-induced loss of adipose Hexokinase 2 triggers hyperglycemia |
| Xiong, S., Krishnan, J., Peuss, R. and Rohner, N. | Conference Paper | 2019 | How cavefish adapt to food limited environment? |
| Kasumyan, A.O. and Vinogradskaya, M.I. | Journal Article | 2019 | Palatability of bile substances for fish |
| Simon, N., Fujita, S., Porter, M. and Yoshizawa, M. | Journal Article | 2019 | Expression of extraocular opsin genes and light-dependent basal activity of blind cavefish |
| Gore, A., Swearer, A., Iben, J. and Weinstein, B. | Conference Paper | 2019 | Cavefish: a model to study subcutaneous fat deposition |
| Blin, M. and Rétaux, S. | Journal Article | 2019 | Voir ou sentir, l’histoire d’Astyanax mexicanus |
| Cao, J. and Cheng, X. | Journal Article | 2020 | Molecular evolution and characterization of fish stathmin genes |
| Ingalls, A., Merryman, S., Baumann, D.P. | Conference Paper | 2020 | Length-mass relationship and aggression of Astyanax mexicanus (Mexican cavefish) reared in a laboratory environment |
| Marandel, L., Plagnes-Juan, E., Marchand, M., Callet, T., Dias, K., Terrier, F., Père, S., Vernier, L., Panserat, S. and Rétaux, S. | Journal Article | 2020 | Nutritional regulation of glucose metabolism-related genes in the emerging teleost model mexican tetra surface fish: a first exploration |
| Policarpo, M., Fumey, J., Lafargeas, P., Naquin, D., Thermes, C., Naville, M., Dechaud, C., Volff, J.-M., Cabau, C., Klopp, C., Rask Møller, P., Bernatchez, L., García-Machado, E., Rétaux, S. and Casane, D. | Journal Article | 2020 | Contrasted gene decay in subterranean vertebrates: insights from cavefishes and fossorial mammals |
| Ma, L., Gore, A.V., Castranova, D., Shi, J., Ng, M., Tomins, K.A., van der Weele, C.M., Weinstein, B.M. and Jeffery, W.R. | Journal Article | 2020 | A hypomorphic cystathionine ß-synthase gene contributes to cavefish eye loss by disrupting optic vasculature |
| Warren, W.C., Boggs, T.E., Borowsky, R., Carlson, B.M., Ferrufino, E., Gross, J.B., Hillier, L., Hu, Z., Keene, A.C., Kenzior, A., et al. | Journal Article | 2020 | A chromosome level genome of Astyanax mexicanus surface fish for comparing population-specific genetic differences contributing to trait evolution |
| Peuss, R., Box, A.C., Chen S., Wang Y., Tsuchiya D., Persons J. L., . . . and Rohner N. | Journal Article | 2020 | Adaptation to low parasite abundance affects immune investment and immunopathological responses of cavefish |
| Yoffe, M., Patel, K., Palia, E., Kolawole, S., Streets, A. Haspel, G. and Soares, D. | Journal Article | 2020 | Morphological malleability of the lateral line allows for surface fish (Astyanax mexicanus) adaptation to cave environments |
| Powers, A.K., Berning, D.J. and Gross, J.B. | Journal Article | 2020 | Parallel evolution of regressive and constructive craniofacial traits across distinct populations of Astyanax mexicanus cavefish. |
| Espinasa, L., Ornelas-García, C.P., Legendre, L., Rétaux, S., Best, A., Gamboa-Miranda, R., Espinosa-Pérez, H. and Sprouse, P. | Journal Article | 2020 | Two new localities of Astyanax cavefish plus revision of its biogeography |
| Ingalls, A., Merryman, S., Baumann, D.P. | Conference Paper | 2020 | Non-invasive measurement of cortisol in Astyanax mexicanus (Mexican tetra) as it relates to environmental stressors |
| Blin, M., Fumey, J., Lejeune, C., Policarpo, M., JLeclercq, J., Père, S., Torres-Paz, J., Pierre, C., Imarazene, B. and Rétaux, S. | Journal Article | 2020 | Diversity of olfactory responses and skills in Astyanax mexicanus cavefish populations inhabiting different caves |
| Cutlip, C. | Web Page | 2020 | Gene found that causes eyes to wither in cavefish |
| Espinasa, L., Ornelas-García,C. P., Legendre, L., Rétaux, S., Best, A., Gamboa-Miranda, R., Espinosa-Pérez, H., and Sprouse, P. | Journal Article | 2020 | Two new localities of Astyanax cavefish plus revision of its biogeography |
| Medley, J.K., Persons, J., Peuß, R., Olsen, L., Xiong, S. and Rohner, N. | Journal Article | 2020 | Untargeted metabolomics of the cavefish Astyanax mexicanus reveals the basis of metabolic strategies in adaptation to extreme conditions |
| Pierre, C., Pradère, N., Froc, C., Ornelas-García, P., Callebert, J. and Rétaux. S. | Journal Article | 2020 | A mutation in monoamine oxidase (MAO) affects the evolution of stress behavior in the blind cavefish Astyanax mexicanus |
| Minchin, J.E.N. | Journal Article | 2020 | Adaptation of blind cavefish to nutrient poor environments: uncovering diverse new mechanisms that regulate body fat levels |
| McGaugh, S.E., Kowalko, J., Duboue, E.R., Lewis, P., Franz-Odendaal, T.A., Rohner, N., Gross, J.B. and Keene, A.C. | Journal Article | 2020 | Dark world rises: The emergence of cavefish as a model for the study of evolution, development, behavior, and disease |
| Wilkens, H. | Journal Article | 2020 | The role of selection in the evolution of blindness in cave fish |
| Rohner, N. | Journal Article | 2020 | In the spotlight—Early career researcher |
| Terán, G.E., Benitez, M.F. and Mirande, J.M. | Journal Article | 2020 | Opening the Trojan horse: phylogeny of Astyanax, two new genera and resurrection of Psalidodon (Teleostei: Characidae) |
| Patch, A., Paz, A., Holt, K., Duboue, E., Kowalko, J., Keene, A. and Fily, Y. | Journal Article | 2020 | Analysis of social swimming dynamics in the Mexican cavefish |
| Phelps, A.N., Luc, H.M. and Gross, J.B. | Conference Paper | 2020 | Comparative developmental expression of neural crest genes in the blind Mexican cavefish, Astyanax mexicanus |
| Jeffery, W.R. | Journal Article | 2020 | Astyanax surface and cave fish morphs |
| Kowalko, J.E., Franz‐Odendaal, T.A. and Rohner, N. | Journal Article | 2020 | Introduction to the special issue—cavefish—adaptation to the dark |
| Kowalko, J.E., Franz-Odendaal, T.A. and Rohner, N. | Journal Article | 2020 | Cavefish special issue |
| Mercado-Silva, N., Ornelas-Garcia, C.P., Schmitter-Soto, J.J., Gidmark, N.J. and Simons, A.M. | Book Section | 2020 | Characidae: Characins |
| Riddle, M.R., Aspiras, A.C., Damen, F., Hutchinson, J.N., Chinnapen, D.J.F., Tabin, J. and Tabin, C.J. | Journal Article | 2020 | Genetic architecture underlying changes in carotenoid accumulation during the evolution of the blind Mexican cavefish, Astyanax mexicanus |
| Ornelas, P., Gonzalez, E.G., Tautz, D. and Doadrio, I. | Journal Article | 2020 | Lack of genetic differentiation between two sympatric species of Astyanax (Characidae:Teleostei) in Lake Catemaco, Mexico |
| Warren, M.L. and Burr, B.M. | Book | 2020 | Freshwater fishes of North America. Volume 2 Characidae to Poeciliidae |
| Torres-Paz, J. and Rétaux, S. | Journal Article | 2020 | Embryological manipulation to probe early evo-devo in the fish Astyanax mexicanus |
| Chin, J.S.R., Loomis, C.L., Albert, L.T., Medina‐Trenche, S., Kowalko, J., Keene, A.C. and Duboué, E.R. | Journal Article | 2020 | Analysis of stress responses in Astyanax larvae reveals heterogeneity among different populations |
| Gallman, K., Fortune, E., Rivera, D. and Soares, D. | Journal Article | 2020 | Differences in behavior between surface and cave Astyanax mexicanus may be mediated by changes in catecholamine signaling |
| Franz-Odendaal, T.A. | Journal Article | 2020 | In the spotlight—Established researcher |
| Krishnan, J., Persons, J.L., Peuß, R., Hassan, H., Kenzior, A., Xiong, S., Olsen, L., Maldonado, E., Kowalko, J.E. and Rohner, N. | Journal Article | 2020 | Comparative transcriptome analysis of wild and lab populations of Astyanax mexicanus uncovers differential effects of environment and morphotype on gene expression |
| Ma, L., Ng, M., van der Weele, C.M., Yoshizawa, M. and Jeffery, W.R. | Journal Article | 2020 | Dual roles of the retinal pigment epithelium and lens in cavefish eye degeneration |
| Kowalko, J.E. | Journal Article | 2020 | In the spotlight—Early career researcher |
| Jandzik, D. and Stock, D.W. | Journal Article | 2020 | Differences in developmental potential predict the contrasting patterns of dental diversification in characiform and cypriniform fishes |
| Riddle, M.R., Aspiras, A.C., Gaudenz, K., Peuß, R., Sung, J.Y., Martineau, B., Peavey, M., Box, A.C., Tabin, J.A., McGaugh, S., Borowsky, R., Tabin, C.J. and Rohner, N. | Journal Article | 2020 | Author Correction: Insulin resistance in cavefish as an adaptation to a nutrient-limited environment |
| Gross, J.B. and Powers, A.K. | Journal Article | 2020 | A natural animal model system of craniofacial anomalies: The blind Mexican cavefish |
| Sifuentes‐Romero, I., Ferrufino, E., Thakur, S., Laboissonniere, L.A., Solomon, M., Smith, C.L., Keene, A.C., Trimarchi, J.M. and Kowalko, J.E. | Journal Article | 2020 | Repeated evolution of eye loss in Mexican cavefish: Evidence of similar developmental mechanisms in independently evolved populations |
| Espinasa, L., Ornelas-García, C.P., Legendre, L., Rétaux, S., Best, A., Gamboa-Miranda, R., Espinosa-Pérez, H. and Sprouse, P. | Journal Article | 2020 | Discovery of two new Astyanax cavefish localities leads to further understanding of the species biogeography |
| Paz, A., McDole, B., Kowalko, J.E., Duboue, E.R. and Keene, A.C. | Journal Article | 2020 | Evolution of the acoustic startle response of Mexican cavefish |
| McGaugh, S.E., Passow, C.N., Jaggard, J.B., Stahl, B.A. and Keene, A.C. | Journal Article | 2020 | Unique transcriptional signatures of sleep loss across independently evolved cavefish populations. |
| Sears, C.R., Boggs, T.E. and Gross, J.B. | Journal Article | 2020 | Dark‐rearing uncovers novel gene expression patterns in an obligate cave‐dwelling fish |
| Ma, L., Ng, M., Shi, J., Gore, A.V., Castranova, D., Weinstein, B.M. and Jeffery, W.R. | Journal Article | 2020 | Evolutionary changes in left-right visceral asymmetry in Astyanax cavefish |
| Emam, A., Yoffe, M., Cardona, H. and Soares, D. | Journal Article | 2020 | Retinal morphology in Astyanax mexicanus during eye degeneration |
| Policarpo, M., Fumey, J., Lafargeas, P., Naquin, D., Thermes, C., Naville, M., Dechaud, C., Volff, J.-M., Cabau, C., Klopp, C., Rask Møller, P., Bernatchez, L., García-Machado, E., Rétaux, S. and Casane, D. | Journal Article | 2020 | Contrasted gene decay in subterranean vertebrates: insights from cavefishes and fossorial mammals |
| Bilandžija H., Hollifield B., Steck M., Meng G., Ng M., Koch A. D, Gračan, R., Ćetković, H., Porter, M.L., Renner, K.J. and Jeffery, W. | Journal Article | 2020 | Phenotypic plasticity as a mechanism of cave colonization and adaptation |
| Pierre, C. | Thesis | 2020 | Conséquences fonctionnelles, comportementales et adaptatives d’une mutation de la MAO (MonoAmine Oxydase) chez le poisson cavernicole aveugle Astyanax mexicanus |
| Jaggard, J.B., Lloyd, E., Yuiska, A., Patch, A., Fily, Y., Kowalko, J.E., Appelbaum, L., Duboue, E.R. and Keene, A.C. | Journal Article | 2020 | Cavefish brain atlases reveal functional and anatomical convergence across independently evolved populations |
| Gimhani, N. and Atukorallaya, D. | Journal Article | 2020 | Influence of the eye on neurocranium development; Insights from Mexican cavefish (Astyanax mexicanus) |
| Krishnan, J., Seidel, C.W., Zhang, N., Van Campen, J., Peuß, R., Xiong, S., Kenzior, A., Li, H., Conaway, J.W. and Rohner, N. | Journal Article | 2020 | Genome-wide analysis of cis-regulatory changes in the metabolic 1 adaptation of cavefish |
| Maldonado, E., Rangel‐Huerta, E., Rodriguez‐Salazar, E., Pereida‐Jaramillo, E. and Martínez‐Torres, A. | Journal Article | 2020 | Subterranean life: Behavior, metabolic, and some other adaptations of Astyanax cavefish |
| Chin, J.S.R., Loomis, C.L., Albert, L.T., Medina-Trenche, S., Kowalko, J., Keene, A.C. and Duboué, E.R. | Journal Article | 2020 | Analysis of stress responses in Astyanax larvae reveals heterogeneity among different populations |
| Leclercq, J., Pierre, C., Simon, V. and Blin, M. | Journal Article | 2020 | La génétique évolutive du tétra mexicain Astyanax mexicanus |
| Mack, K.L., Jaggard, J.B., Persons, J.L., Passow, C.N., Stanhope, B.A., Ferrufino, E., Tsuchiya, D., Smith, S.E., Slaughter, B.D., Kowalko, J., Rohner, N., Keene, A.C. and McGaugh, S.E. | Journal Article | 2020 | Repeated evolution of circadian clock dysregulation in cavefish populations |
| Kowalko, J. | Journal Article | 2020 | Utilizing the blind cavefish Astyanax mexicanus to understand the genetic basis of behavioral evolution |
| Riddle, M.R., Damen, F., Aspiras, A., Tabin, J.A., McGaugh, S. and Tabin, C.J. | Journal Article | 2020 | Evolution of gastrointestinal tract morphology and plasticity in cave-adapted Mexican tetra, Astyanax mexicanus |
| Powers, A.K., Boggs, T.E.and Gross, J.B. | Journal Article | 2020 | An asymmetric genetic signal associated with mechanosensory expansion in cave-adapted fish |
| Peuß, R., Box, A.C., Chen, S., Wang, Y., Tsuchiya, D., Persons, J.L., Kenzior, A., Maldonado, E., Krishnan, J., Scharsack, J.P., Slaughter, B.D. and Rohner, N. | Journal Article | 2020 | Adaptation to low parasite abundance affects immune investment and immunopathological responses of cavefish. |
| Pierre, C., Pradère, N., Froc, C., Ornelas-García, P., Callebert, J. and Rétaux, S. | Journal Article | 2020 | A mutation in monoamine oxidase (MAO) affects the evolution of stress behavior in the blind cavefish Astyanax mexicanus |
| Moran, R.L., Jaggard, J.B., Roback, E.Y., Rohner, N., Kowalko, J.E., Ornelas-García, C.P., McGaugh, S.E. and Keene, A.C. | Journal Article | 2021 | Hybridization underlies localized trait evolution in cavefish |
| Warren, W.C., Boggs, T.E., Borowsky, R., Carlson, B.M., Ferrufino, E., Gross, J.B., Hillier, L., Hu, Z., Keene, A.C., Kenzior, A. and Kenzior, A. and Kowalko, J.E. | Journal Article | 2021 | A chromosome-level genome of Astyanax mexicanus surface fish for comparing population-specific genetic differences contributing to trait evolution |
| Oliva, C., Hinz, N.K., Robinson, W., Barrett Thompson, A.M., Booth, J., Crisostomo, L.M., Zanineli, S., Tanner, M., Lloyd, E., O’Gorman, M., McDole, B., Paz, A., Kozol, R. et al. | Journal Article | 2021 | Characterizing the genetic basis of trait evolution in the Mexican cavefish |
| Mack, K.L., Jaggard, J.B., Persons, J.L., Roback, E.Y., Passow, C.N., Stanhope, B.A., Ferrufino, E., Tsuchiya, D. Smith, S.E., Slaughter, B.D. et al. | Journal Article | 2021 | Repeated evolution of circadian clock dysregulation in cavefish populations |
| Devos, L., Agnès, F., Edouard, J., Simon, V., Legendre, L., El Khallouki, N., Barbachou, S., Sohm, F. and Rétaux, S. | Journal Article | 2021 | Eye morphogenesis in the blind Mexican cavefish |
| Gimhani, N. and Atukorallaya, D. | Journal Article | 2021 | Comparative analysis of the parasphenoid bone development in Mexican tetra (Astyanax mexicanus) |
| Xiong, S., Wang, W., Kenzior, A., Olsen, L., Krishnan, J., Persons, J., Medley, K., Peuß, R., Wang, Y., Chen, S., Zhang, N., Thomas, N., Miles, J.M., Sánchez Alvarado, A. and Rohner, N. | Journal Article | 2021 | Enhanced lipogenesis through Pparγ helps cavefish adapt to food scarcity |
| Torres-Paz, J. and Rétaux, S. | Journal Article | 2021 | Pescoids and chimeras to probe early evo-devo in the fish Astyanax mexicanus |
| Boggs, T. and Gross, J. | Journal Article | 2021 | Reduced oxygen as an environmental pressure in the evolution of the blind Mexican cavefish |
| Imarazene, B., Du, K., Beille, S., Jouanno, E., Feron, R., Pan, Q., Torres-Paz J., Lopez-Roques, C., Castinel,A., Gil, L., Kuchly, C., Donnadieu, C., Parrinello,H., Journot, L., Cabau, C., Zahm, M., Klopp, C., Pavlica, T., Al-Rikabi, A., Liehr, T., Sima | Journal Article | 2021 | A supernumerary ‘‘B-sex’’ chromosome drives male sex determination in the Pachon cavefish, Astyanax mexicanus |
| Agnès, F., Torres-Paz, J., Michel, P. and Rétaux, S. | Journal Article | 2021 | A 3D molecular map of the cavefish neural plate illuminates eyefield organization and its borders in vertebrates |
| Ma, L., Ng, M., Shi, J., Gore, A.V., Castranova, D., Weinstein, B.M. and Jeffery, W.R. | Journal Article | 2021 | Maternal control of visceral asymmetry evolution in Astyanax cavefish |
| Tanvir, Z., Rivera, D., Severi, K.E., Haspel, G. and Soares, D. | Journal Article | 2021 | Evolutionary and homeostatic changes in morphology of visual dendrites of Mauthner cells in Astyanax blind cavefish. |
| Iwashita, M. and Yoshizawa, M. | Journal Article | 2021 | Social-like responses are inducible in asocial Mexican cavefish despite the exhibition of strong repetitive behaviour |
| Ma, L., Dessiatoun, R., Shi, J. and Jeffery, W.R. | Journal Article | 2021 | Incremental temperature changes for maximal breeding and spawning in Astyanax mexicanus. |
| Riddle, M.R., Aspiras, A., Damen, F., McGaugh, S., Tabin, J.A. and Tabin, C.J. | Journal Article | 2021 | Genetic mapping of metabolic traits in the blind Mexican cavefish reveals sex‑dependent quantitative trait loci associated with cave adaptation |
| Eschmeyer, W.N. and Van der Laan, R. and Fricke, R. | Web Page | 2021 | Eschmeyer's Catalog of Fishes: Genera, Species, References |
| Borowsky, R. | Journal Article | 2021 | Evolution: The genetics of trait evolution in cavefish |
| O'Gorman, M., Thakur, S., Imrie, G., Moran, R.L., Choy, S., Sifuentes-Romero, I., Bilandžija, H., Renner, K.J., Duboué, E., Rohner, N., McGaugh, S.E., Keene, A.C. and Kowalko, J.E. | Journal Article | 2021 | Pleiotropic function of the oca2 gene underlies the evolution of sleep loss and albinism in cavefish |
| Zhao, H., Li, H., Du, J., Di Mauro, G., Lungu-Mitea, S., Geyer, N., Vallone, D., Bertolucci, C. and Foulkes, N.S. | Journal Article | 2021 | Regulation of ddb2 expression in blind cavefish and zebrafish reveals plasticity in the control of sunlight-induced DNA damage repair |
| Wilkens, H. | Journal Article | 2021 | Variability and the primacy of the genotype |
| Garita-Alvarado, C.A., Garduño-Sánchez, M., Barluenga, M. and Ornelas-García, C.P. | Journal Article | 2021 | Genetic and ecomorphological divergence between sympatric Astyanax morphs from Central America |
| Kistner, A.R., Enriquez, M.S., Michels, N.O. and Mensinger, A.F. | Conference Paper | 2021 | Behavioral differences in sound detection in recently diverged cave and surface forms of Astyanax mexicanus |
| Policarpo, M., Laurenti, P., García-Machado, E., Metcalfe, C., Rétaux, S. and Casane, D. | Journal Article | 2021 | Genomic evidence that blind cavefishes are not wrecks of ancient life |
| Espinasa, L., Heintz, C., Rétaux, S., Yoshisawa, M., Agnès, F., Ornelas-Garcia, P. and Balogh-Robinson, R. | Journal Article | 2021 | Vibration Attraction Response (VAB) is a plastic trait in Blind Mexican tetra (Astyanax mexicanus), variable within subpopulations inhabiting the same cave |
| Iwashita, M. and Yoshizawa, M. | Journal Article | 2021 | Social-like responses are inducible in asocial Mexican cavefish despite the exhibition of strong repetitive behavior |
| Garita-Alvarado, C.A., Garduño-Sánchez, M., Barluenga, M. and Ornelas-García, C.P. | Journal Article | 2021 | Genetic and ecomorphological divergence between sympatric Astyanax morphs from Central America |
| Jandzik, D. and Stock, D.W. | Journal Article | 2021 | Differences in developmental potential predict the contrasting patterns of dental diversification in characiform and cypriniform fishes |
| Wilson, E.J., Tobler, M., Riesch, R., Martínez‑García, L. and García‑De León, F.J. | Journal Article | 2021 | Natural history and trophic ecology of three populations of the Mexican cavefish, Astyanax mexicanus |
| Devos, L., Agnès, F., Edouard, J., Simon, V., Legendre, L., El Khallouki, N., Barbachou S., Sohm, F. and Rétaux, S. | Journal Article | 2021 | Eye morphogenesis in the blind Mexican cavefish |
| Potts, H.G., Stockdale,W.T. and Mommersteeg, M.T.M. | Journal Article | 2021 | Unlocking the secrets of the regenerating fish heart: Comparing regenerative models to shed light on successful regeneration. |
| Olsen, L., Thum, E. and Rohner, N. | Journal Article | 2021 | Lipid metabolism in adaptation to extreme nutritional challenges |
| Broadfoot, M.V. | Web Page | 2021 | Stowers Institute amasses menagerie of unconventional organisms to study health and disease |
| Torres-Paz, J. and Retaux, S. | Journal Article | 2021 | Pescoids and chimeras to probe early evo-devo in the fish Astyanax mexicanus |
| Krishnan, J., Seidel, C.W., Zhang, N., VanCampen, J., Peuss, R., Xiong, S., Kenzior, A., Li, H., Conaway, J.W. and Rohner, N. | Journal Article | 2021 | RNA-Seq Analysis of liver cells of Astyanax mexicanus |
| Imarazene, B., Beille, S., Jouanno, E., Branthonne, A., Thermes, V., Thomas, M., Herpin, A., Rétaux, S. and Guiguen, Y. | Journal Article | 2021 | Primordial germ cell migration and histological and molecular characterization of gonadal differentiation in Pachón cavefish Astyanax mexicanus. |
| Pérez-Rodríguez, R., Esquivel-Bobadilla, S., Orozco-Ruíz, A.M., Olivas-Hernández, Jé.L. and García-De León, F.J. | Journal Article | 2021 | Genetic structure and historical and contemporary gene flow of Astyanax mexicanus in the Gulf of Mexico slope: a microsatellite-based analysis |
| Leclercq, J. | Thesis | 2022 | Evolution of the eye in Astyanax mexicanus : contribution of maternal and early developmental regulations |
| Medley, J.K., Persons, J., Biswas, T., Olsen, L., Peuß, R., Krishnan, J., Xiong, S. and Rohner, N. | Journal Article | 2022 | The metabolome of Mexican cavefish shows a convergent signature highlighting sugar, antioxidant, and ageing-related metabolites |
| Leclercq, J., Torres-Paz, J. and Rétaux, S. | Conference Paper | 2022 | Evolution of gene expression regulation in blind cavefish embryos |
| Agnès, F., Torres-Paz, J., Michel, O. and Rétaux, S. | Journal Article | 2022 | A 3D molecular map of the cavefish neural plate illuminates eye-field organization and its borders in vertebrates |
| Lloyd, E., McDole, B., Privat, M., Jaggard, J.B., Duboue, E.R., Sumbre, G. and Keene, A.C. | Journal Article | 2022 | Blind cavefish retain functional connectivity in the tectum despite loss of retinal input |
| Lloyd, E., McDole, B., Privat, M., Jaggard, J.B., Duboué, E., Sumbre, G. and Keene, A. | Conference Paper | 2022 | Blind cavefish retain functional connectivity in the tectum despite loss of retinal input |
| Nwosu, U. | Thesis | 2022 | Regulation of activity patterns in Astyanax mexicanus cavefifish |
| Boggs, T. and Gross, J.B. | Conference Paper | 2022 | Evidence of adaptation to hypoxic subterranean waters in Astyanax mexicanus. |
| Agnès, F., Torres-Paz, J., Michel, P. and Rétaux, S. | Journal Article | 2022 | A 3D molecular map of the cavefish neural plate illuminates eye-field organization and its borders in vertebrates |
| Garduno-Sanchez, M., Hernandez-Lozano, J., Moran, R., Miranda-Gamboa, R., Gross, J., Rohner, N., Elliott, W.R., Miller, J., Lozano-Vilano, L., McGaugh, S. and Ornelas-Garcia, C. | Journal Article | 2022 | Phylogeographic relationships and morphological evolution between cave and surface Astyanax mexicanus populations (De Fillipi 1853) (Actinopterygii, Characidae) |
| Krishnan, J., Wang, Y., Kenzior, O., Hassan, H., Olsen, L., Tsuchiya, D., Kenzior, A., Peuß, R., Xiong, S., Wang, Y., Zhao, C. and Rohner, N. | Journal Article | 2022 | Liver-derived cell lines from cavefish Astyanax mexicanus as an in vitro model for studying metabolic adaptation |
| Rodriguez-Morales, R., Gonzalez-Lerma, P., Yuiska, A., Heon Han, J., Guerra, Y., Crisostomo, L., Keene, A.C., Duboue, E.R. and Kowalko, J.E. | Journal Article | 2022 | Convergence on reduced aggression through shared behavioral traits in multiple populations of Astyanax mexicanus |
| Bhatia, V., de Jesus, V.C., Shaik, F.A., Jaggupilli, A., Singh, N., Chelikani, P. and Atukorallaya, D. | Journal Article | 2022 | Extraoral expression and characterization of bitter taste receptors in Astyanax mexicanus (Mexican tetra fish) |
| Powers, A., Berning, D., Boggs, T., Hamm, A. and Gross, J. | Conference Paper | 2022 | Towards an understanding of the developmental and genetic attributes of cranial variation in Astyanax cavefish |
| Pennington, A. | Thesis | 2022 | Bioinformatics analyses of new genes of focus in the research of autism with dogs, fats, and a variety of other model organisms |
| van der Weele, C. and Jeffery, W.R. | Conference Paper | 2022 | Cavefish develop more erythrocytes and overexpress hypoxia Inducible genes as a response to hypoxic environments |
| Rodríguez-Ballesteros, V., Mendoza-Garfias, B., Ulloa-Arvizu, R., Balcazar, A. and Ornelas-García, C.P. | Journal Article | 2022 | Morphological description of gametes in cave and surface populations of Astyanax mexicanus (De Filippi, 1853) |
| Rodriguez-Morales, R., Gonzalez-Lerma, P., Yuiska, A., Heon Han, J., Guerra, Y., Crisostomo, L., Keene, A.C., Duboue, E.R. and Kowalko, J. | Journal Article | 2022 | Convergence on reduced aggression through shared behavioral traits in multiple populations of Astyanax mexicanus |
| Powers, A.K and Tabin, C. | Conference Paper | 2022 | The genetic basis underlying jaw size differences in cavefish |
| Oliva, C., Hinz, N.K., Robinson, W., Barrett Thompson, A.M., Booth, J., Crisostomo, L.M., Zanineli, S., Tanner, M., Lloyd, E., O’Gorman, M., McDole, B., Paz, A., Kozol, R. et al. | Journal Article | 2022 | Characterizing the genetic basis of trait evolution in the Mexican cavefish |
| Olsen, L., Gluesenkamp, A., Mager, E., Coughlin, D. and Rohner, N. | Conference Paper | 2022 | Metabolic reprogramming underlies cavefish muscular endurance despite selective loss of muscle mass and contractility |
| Fernandes, V.F.L., Glaser, Y., Iwashita, M. and Yoshizawa, M. | Journal Article | 2022 | Evolution of left–right asymmetry in the sensory system and foraging behavior during adaptation to food‑sparse cave environments |
| Berning, D. and Gross, J.B. | Conference Paper | 2022 | Developmental timing and genetic architecture of external taste expansion in the Mexican tetra, Astyanax mexicanus |
| Agnes, F., Torres-Paz, J., Michel, P. and Rétaux, S. | Conference Paper | 2022 | A 3D molecular map of the cavefish neural plate illuminates eyefield organization and its borders in vertebrates |
| Duboue, E.R., Kowalko, J.E. and Keene, A.C. | Journal Article | 2022 | Course‐based undergraduate research experiences (CURES) as a pathway to diversify science |
| Callier, V. | Journal Article | 2022 | Blind Cave Fish May Trade Color for Energy. Pasty cave fish seem to repurpose a melanin-making molecule to better survive famine |
| Yoshizawa, M., Balmilero-Unciano, R., Valdez, C., Lactaoen, K., Kato, J., Ito, M., Clarke, K. and Iwashita, M. | Conference Paper | 2022 | Plasticity in the expression of social affinity under the environmental interventions: spatial and dietary |
| Legendre, L., Rode, J., Germon, I., Pavie, M., Quiviger, C., Policarpo, M., Leclercq, J., Père, S., Fumey, J., Hyacinthe, C., Ornelas-García, P., Espinasa, L., Rétaux, S. and Casane, D. | Journal Article | 2022 | Genetic identification and reiterated captures suggests that the Astyanax mexicanus El Pachón cavefish population is closed and declining |
| Goes, C.A.G., dos Santos, R.Z., Aguiar, W.R.C., Alves, D.C.V., Silva, D.M.Z.dA., Foresti, F., Oliveira, C., Utsunomia, R. and Porto-Foresti, F. | Journal Article | 2022 | Revealing the satellite DNA history in Psalidodon and Astyanax Characid fish by comparative satellitomics |
| Jeffery, W.R. Ma, L., Ng, M., Shi, J. and Gore, A.V. | Conference Paper | 2022 | Control of visceral asymmetry in Astyanax development: Sonic Hedgehog and the left-right organizer |
| Kuhn, J., Azari, S. and Volkoff, H. | Journal Article | 2022 | Effects of temperature on food intake and the expression of appetite regulators in three Characidae fish: The black-skirted tetra (Gymnocorymbus ternetzi), neon tetra (Paracheirodon innesi) and Mexican cavefish (Astyanax mexicanus) |
| Potts, H.G., Lemieux, M.E., Rice, E.S., Warren,W., Choudhury, R.P. and Mommersteeg, M.T.M. | Journal Article | 2022 | Discordant genome assemblies drastically alter the interpretation of single-cell RNA sequencing data which can be mitigated by a novel integration method |
| Xiong, S., Wang, W., Kenzior, A., Olsen, L., Krishnan, J., Persons, J., Medley, K., Peuß, R., Wang, Y., Chen, S., Zhang, N., Thomas, N., Miles, J. M., Alvarado, A. S. and Rohner, N. | Journal Article | 2022 | Enhanced lipogenesis through Pparγ helps cavefish adapt to food scarcity |
| Espinasa, L. and Diamant, R. | Conference Paper | 2022 | Laterality in foraging behavior, reproductive seasonality, and other results from several multi-year, longitudinal studies in the field |
| Paz, A., Holt, K., Duboué, E., Fily, Y. and Kowalko, J. | Conference Paper | 2022 | The developmental trajectory of social behavior in the Mexican cavefish |
| Espinasa, L., Collins, E., Ornelas García, C.P., Rétaux, S., Rohner, N. and Rutkowski, J. | Journal Article | 2022 | Divergent evolutionary pathways for aggression and territoriality in Astyanax cavefish |
| Choy, S., Polyakov, E., Thakur, S., Lloyd, E., Keene, A. and Kowalko, J. | Conference Paper | 2022 | Uncovering the role of oca2 in the evolution of prey capture in cavefish |
| Vogt, G. | Book Section | 2022 | Epigenetics and phenotypic plasticity in animals |
| Maderspacher, F. | Journal Article | 2022 | White, fat and blind — Economy and evolution in caves |
| Moran, R.L., Jaggard, J.B., Roback, E.Y., Kenzior, A., Rohner, N., Kowalko, J.E., Ornelas-García, C.P., McGaugh, S.E. and Keene, A.C. | Conference Paper | 2022 | Hybridization underlies localized trait evolution in cavefish |
| Berning, D. | Thesis | 2022 | Developmental timing and genetic architecture of external taste expansion in the blind Mexican cavefish, Astyanax mexicanus |
| Lunghi, E. and Bilandžija, H. | Conference Paper | 2022 | Average telomere length in cave vs surface Astyanax mexicanus |
| Rodríguez-Morales, R., Gonzalez, P., Guerra, Y., Crisostomo, L., Pileggi, B., Kimmel, A. and Kowalko, J. | Conference Paper | 2022 | Evolution of aggressive behaviors in the blind Mexican cavefish |
| Tomkins, J.P., Arledge, S. and Guliuzza, R.J. | Journal Article | 2022 | Blind cave fish (Astyanax mexicanus) as a model system for continuous environmental tracking and adaptive engineering |
| Hutton, P., Rodríguez-Morales, R., Ferrufino, E., Lloyd, E., Dubuoé, E.R., Kowalko, J.E. and Keene, A.C. | Conference Paper | 2022 | Preliminary data from a functional screen reveals multiple sleep-regulating genes in A. mexicanus |
| Lesku, J.A. and Rattenborg, N.C. | Journal Article | 2022 | The missing cost of ecological sleep loss |
| Perry, A., McGaugh, S.E., Keene, A.C. and Blackmon, H. | Journal Article | 2022 | CaveCrawler: an interactive analysis suite for cavefish bioinformatics |
| Wilson, E.J., Tobler, M., Riesch, R., Martínez-García, L. and García-De León, F.J. | Conference Paper | 2022 | Natural diet of three populations of the Mexican cavefish, Astyanax mexicanus |
| Santacruz, A., Hernández-Mena, D., Miranda-Gamboa, R.A., Pérez-Ponce de León, G. and Ornelas-Garcíai, P. | Conference Paper | 2022 | Host-parasite interactions after niche change: the macroparasite diversity in the Mexican cavefish Astyanax mexicanus |
| Leal, F. and Tabin, C.J. | Conference Paper | 2022 | Genetic basis of metabolic specializations in the Mexican cavefish |
| Hyacinthe, C. and Tabin, C. | Conference Paper | 2022 | Evolution of temperature preference in Astyanax mexicanus |
| Roberts, R.J.B., Pop, S. and Prieto-Godino, L.L. | Journal Article | 2022 | Evolution of central neural circuits: state of the art and perspectives |
| Hyacinthe, C., Attia, J., Schutz, E., Casane, D. and Rétaux, S. | Journal Article | 2022 | Acoustic signatures in cavefish populations inhabiting different caves |
| Shafer, M.E.R., Sawh, A.N. and Schier, A.F. | Journal Article | 2022 | Gene family evolution underlies cell-type diversification in the hypothalamus of teleosts |
| Tanvir, Z., Rivera, D., Severi, K.E., Haspel, G. and Soares, D. | Conference Paper | 2022 | Evolutionary and homeostatic changes in morphology of visual dendrites of Mauthner cells in Astyanax blind cavefish |
| Espinasa, L., Diamant, R., Mesquita, M., Lindquist, J.M., Powers, A.M. and Helmreich, J. | Journal Article | 2022 | Laterality in cavefish: Left or right foraging behavior in Astyanax mexicanus |
| Lloyd, E., Chouuk, B., Conith, A., Albertson, R.C. and Keene, A.C. | Conference Paper | 2022 | Investigating the evolution and developmental plasticity of sleep and activity patterns in Lake Malawi cichlids |
| Espinasa, L., Rohner, N. and Rétaux, S. | Journal Article | 2022 | Reproductive seasonality of Astyanax mexicanus cavefish |
| Schartl, M. | Conference Paper | 2022 | Sex determination in the Pachón cavefish, Astyanax mexicanus, by B-chromosomes |
| Soares, D. | Conference Paper | 2022 | Evolutionary insights and constraints from the nervous systems and behavior of cavefish |
| Kozol, R.V., Conith, A.J., Yuiska, A., Cree-Newman, A., Tolentino, B., Banesh, K., Paz, A., Lloyd, E., Kowalko, J.E., Keene, A.C., R. Albertson, C. and Duboue, E.R. | Journal Article | 2022 | A brain-wide anatomical analysis suggests structural coevolution of distinct neuroanatomical modules |
| Fily, Y., Patch, A., Paz, A., Holt, K.J., Duboué, E.R., Keene, A.C. and Kowalko, J.E. | Conference Paper | 2022 | Evolution of schooling and exploration strategies in the Mexican tetra fish |
| Boggs, T.E., Friedman, J.S. and Gross, J.B. | Journal Article | 2022 | Alterations to cavefish red blood cells provide evidence of adaptation to reduced subterranean oxygen |
| Mogdans, J. | Book Section | 2022 | The processing of hydrodynamic stimuli with the fish lateral line system |
| Tobler, M. | Conference Paper | 2022 | Extreme environments, natural complexity, and the predictability of evolution |
| Rodriguez-Santiago, M., Jordan, A. and Hofmann, H.A. | Journal Article | 2022 | Neural activity patterns differ between learning contexts in a social fish |
| Anonymous | Web Page | 2022 | How blind fish find their way in pitch-black caves |
| Gross, J.B., and Powers, A.K. | Journal Article | 2022 | Reinterpreting the work of Charles Breder: Sensory neuromasts and orbital skeleton variation in eyeless Astyanax cavefish |
| Olsen, L., Levy, M., Medley, J.K., Hassan, H., Alexander, R., Wilcock, E., Yi, K., Florens, L., McKinney, S., Peuß, R., Persons, J., Kenzior, A., Maldonado, E., Gluesenkamp, A. et al. | Journal Article | 2022 | Metabolic reprogramming underlies cavefish muscular endurance despite loss of muscle mass and contractility |
| Lunsford, E.T., Paz, A., Keene, A.C. and Liao, J.C. | Journal Article | 2022 | Evolutionary convergence of a neural mechanism in the cavefish lateral line system |
| Leclercq, J., Torres-Paz, J., Policarpo, M., Agnès, F. and Rétaux, S. | Journal Article | 2022 | Evolution of the regulation of developmental gene expression in blind Mexican cavefish |
| Krishnan, J., Wang, Y., Kenzior, O., Huzaifa, H., Olsen, L., Tsuchiya, D., Wang, Y., Zhao, C. and Rohner, N. | Journal Article | 2022 | Liver-derived cell lines from cavefish Astyanax mexicanus as an in vitro model for studying metabolic adaptation |
| Reyes Pérez, R.I. | Thesis | 2022 | Evolutionary development of skull morphology in the cavefish Astyanax mexicanus |
| Sumbre, G. | Conference Paper | 2022 | Principles of functional circuit connectivity: Insights from the zebrafish optic tectum |
| Patch, A., Paz, A., Holt, K.J., Duboué, E.R., Keene, A.C., Kowalko, J.E. and Fily, Y. | Journal Article | 2022 | Kinematic analysis of social interactions deconstructs the evolved loss of schooling behavior in cavefish |
| Anonymous | Journal Article | 2022 | How blind cavefishes found themselves up against the wall |
| Rice, E. Warren, W.C., Carroll, R., Keene, A. and Rohner, N. | Conference Paper | 2022 | The evolution of structural change along a surface to cavefish genome trajectory |
| Lunsford, E.T. | Thesis | 2022 | Mechanisms and evolution of sensory feedback and signal transduction in lateral line hair cells |
| Porfiri, M., Zhang, P. and Peterson, S.D. | Journal Article | 2022 | Hydrodynamic model of fish orientation in a channel flow |
| Jeffery, W. | Conference Paper | 2022 | Environmental hypoxia is a driver of Sonic Hedgehog-dependent troglomorphic evolution in Astyanax cavefish |
| Baumann, D.P. and Ingalls, A. | Book Section | 2022 | Mexican tetra (Astyanax mexicanus): biology, husbandry, and experimental protocols |
| Stanton, D., Justin, H.S. and Reitzel, A.M. | Journal Article | 2022 | Step in time: Conservation of circadian clock genes in animal evolution |
| Thakur, S., Tuyet Tra, N. and Cummings, M. | Conference Paper | 2022 | Understanding coping styles in a fish species with alternative reproductive tactics |
| Perry, A. McGaugh, S.E., Keene, A.C. and Blackmon, H. | Conference Paper | 2022 | CaveCrawler: An interactive analysis suite for cavefish bioinformatics |
| Páscoa, I., Fonseca, E., Ferraz, R., Machado, A.M., Conrado, F., Ruivo, R., Cunha, I. and Castro, L.F.C. | Journal Article | 2022 | The preservation of PPARg genome duplicates in some Teleost tineages: Insights into lipid metabolism and xenobiotic exploitation |
| van der Weele C.M. and Jeffery, W.R. | Journal Article | 2022 | Cavefish cope with environmental hypoxia by developing more erythrocytes and overexpression of hypoxia-inducible genes |
| Silva. P., Zavareh, P.A. and Atukorala, D. | Book Section | 2022 | Teleost fish as model animals to understand alcohol teratology |
| Hendrickson, D. | Conference Paper | 2022 | Not yet known |
| Baratti, G., Potrich, D., Lee, S.A., Morandi-Raikova, A. and Sovrano, V.A. | Journal Article | 2022 | The geometric world of fishes: A synthesis on spatial reorientation in Teleosts |
| Biswas, T., Paulson, A., Hall, K, and Rohner, N. | Conference Paper | 2022 | Fish(ing) in the Hi(gh)-C(sea) |
| Kemeny, R. | Web Page | 2022 | Blind Mexican cave fish are developing cave-specific accents. The Mexican tetra has evolved to live in a number of dark caves – and now we know that the fish in each cave use clicks to communicate in distinct ways |
| Enriquez, M.S. | Thesis | 2022 | Investigating rapid divergence of sensory systems between satellite populations of the Mexican tetra (Astyanax mexicanus) |
| Perry, A., McGaugh, S.E., Keene, A.C. and Blackmon, H. | Web Page | 2022 | CaveCrawler |
| Lunsford, E.T., Paz, A., Keene, A.C. and Liao, J.C. | Journal Article | 2022 | Evolutionary convergence of a neural mechanism in the cavefish lateral line system |
| Fernandes, V.F.L., Espinasa, L., Glaser, Y., Iwashita, M., Sadowski, P. and Yoshizawa, M. | Conference Paper | 2022 | Diversity of the left-right asymmetry evolution in the sensory system and the behavior output of Astyanax mexicanus |
| Bronner, M. | Conference Paper | 2022 | Not yet known |
| Smith, D.G. and Bowman, I.A. | Journal Article | 2022 | The long journey of Curt Kosswig |
| Riddle, M., Guerra, D.P., Aspiras, A., Chinnapen, D., Damen, F., Hutchinson, J.N., McGaugh, S., Tabin, J. and Tabin, C. | Conference Paper | 2022 | Evolution of provitamin A assimilation in Astyanax mexicanus |
| Moran, R.L., Jaggard, J.B., Roback, E.Y., Kenzior, A., Rohner, N., Kowalko, J.E., Ornelas-García, C.P., McGaugh, S.E. and Keene, A.C. | Journal Article | 2022 | Hybridization underlies localized trait evolution in cavefish |
| Lesser, E. | Web Page | 2022 | Cavefish focus on which way the water flows |
| Martik, M. | Conference Paper | 2022 | Reactivation of the embryonic neural crest gene program during zebrafish cardiac regeneration |
| Powers, A.K., Hyacinthe, C., Riddle, M., Kim, Y.K., Amaismeier, A., Thiel, K., Martineau, B., Ferrante, E., Moran, R., McGaugh, S., Boggs, T., Gross, J.B. and Tabin, C.J. | Journal Article | 2022 | Genetic mapping of craniofacial traits in the Mexican tetra reveals loci associated with bite differences between cave and surface fish |
| Pierre, C., Callebert, J., Launay, J.M. and Rétaux, S. | Journal Article | 2022 | The enzymatic and neurochemical outcomes of a mutation in Mexican cavefish MAO reveal teleost-specific aspects of brain monoamine homeostasis |
| Krishnan, J., Seidel, C.W., Zhang, N., Singh, N.P., VanCampen, J., Peuß, R., Xiong, S., Kenzior, A., Li, H., Conaway. J.W. and Rohner, N. | Journal Article | 2022 | Genome-wide analysis of cis-regulatory changes underlying metabolic adaptation of cavefish |
| Krishnan, J., Seidel, C.W., Zhang, N., Singh, N.P., Van Campen, J., Peuß, R., Xiong, S., Kenzior, A., Li, H., Conaway, J.W. and Rohner, N. | Conference Paper | 2022 | Genome-wide analysis of cis-regulatory changes underlying metabolic adaptation of cavefish |
| Iwashita, M., Tran, A., Wong, M., Lee, R. and Yoshizawa, M. | Journal Article | 2022 | Metabolic shift toward ketosis in asocial cavefish increases social-like collective behavior |
| Rétaux, S. | Journal Article | 2022 | La boîte à outils de l’évolution développementale ou comment les poissons cavernicoles mexicains ont perdu leurs yeux |
| Kozol, R.A., Conith, A.J., Yuiska, A., Cree-Newman, A., Tolentino, B., Banesh, K., Paz, A., Lloyd, E., Kowalko, J.E., Keene, A.C., Albertson, R.C. and Duboue, E.R. | Conference Paper | 2022 | Global and discrete mechanisms of evolution have transformed the shape and volume of cavefish brain regions |
| Retaux, S., Attia, J., Blin, M., Casane, D., Espinasa, L., Hyacinthe, C., Imarazene, B., Leclercq, J., Legendre, L., Père, S., Pierre, C., Policarpo, M., Torres-Paz, J., and Simon, V. | Conference Paper | 2022 | Ten years of cavefish field studies: what have we learned? |
| Borowsky, R. and Chen, H. | Journal Article | 2022 | Mutagenesis alters sperm swimming velocity in Astyanax |
| Espinasa, L. and Pech, A. | Journal Article | 2023 | Biogeographical affinities of the aquatic community of Refugio Cave, a newly discovered Astyanax cave |
| Di Lorenzo, T., Avramov, M., Galassi, D.M.P., Iepure, S., Mammola, S., Reboleira, A.S.P.S and Hervant, F. | Book Section | 2023 | Chapter 20. Physiological tolerance and ecotoxicological constraints of groundwater fauna |
| Santacruz, A., Hernández-Mena, D., Miranda-Gamboa, R., Pérez-Ponce De León, G. and Ornelas-García, P. | Journal Article | 2023 | Host-parasite interactions in perpetual darkness: Macroparasite diversity in the cavefish Astyanax mexicanus |
| Pavlova, V.V. and Krylov, V.V. | Journal Article | 2023 | Cavefishes in chronobiological research: A narrative review. |
| Garduño-Sánchez, M., Hernández-Lozano, J., Moran, R.L., Miranda-Gamboa, R., Gross, J.B., Rohner, N., Elliott, W.R., Miller, J., Lozano-Vilano, L., McGaugh, S.E. and Ornelas-García, C.P. | Journal Article | 2023 | Phylogeographic relationships and morphological evolution between cave and surface Astyanax mexicanus populations (De Filippi 1853) (Actinopterygii, Characidae) |
| Casane, D., Saclier, N., Policarpo, M., François, C. and Lefébure, T. | Book Section | 2023 | Chapter 17. Evolutionary genomics and transcriptomics in groundwater animals |
| Lloyd, E., Privat, M., Sumbre, G., Duboue, E.R. and Keene, A.C. | Journal Article | 2023 | A protocol for whole-brain Ca2+ imaging in Astyanax mexicanus, a model of comparative evolution |
| Policarpo, M., Legendre, L., Germon, I., Lafargeas, P., Espinasa, L., Rétaux, S. and Casane, D. | Journal Article | 2023 | The nature and distribution of non-functional alleles suggest only two independent events at the origins of Astyanax mexicanus cavefish populations. |
| Zhang, J.H., Long, R., Jing, Y.Y., Zhang, P., Xu, Y., Xiong, W., Zhu, Y.Q. and Luo, Y.P. | Journal Article | 2023 | Loss of behavioral stress response in blind cavefish reduces energy expenditure |
| Garduño-Sánchez, M.A.A., de Jesus-Bonilla, V., Perea, S., Miranda-Gamboa, R., Herrera- García, A., de la Maza Benignos, M. and Ornelas-García, C.P. | Journal Article | 2023 | Mitochondrial phylogeography and molecular evolution of the rhodopsin visual pigment in troglobitic populations of Astyanax mexicanus (De Filippi, 1853) |
| Bucher, L. and Gross, J. | Web Page | 2023 | Mexican cavefish living in hypoxic environments exhibit changes in expression of oxygen-transporters |
| Jiménez, A.G., Nash-Braun, E. and Meyers, J.R. | Journal Article | 2023 | White epaxial muscle aerobic and anaerobic potential and muscle fiber structure in surface and cave morphotypes of the Mexican cavefish (Astyanax mexicanus) |
| Borowsky, R.L. | Journal Article | 2023 | Selection maintains the phenotypic divergence of cave and surface fish |
| Lomheim, H.J., Rodas, L.R., Mulla, L., Freeborn, L., Sun, D.A., Sanders, S.A. and Protas, M.E. | Journal Article | 2023 | Transcriptomic analysis of cave, surface, and hybrid samples of the isopod Asellus aquaticus and identification of chromosomal location of candidate genes for cave phenotype evolution |
| Culver, D.C., Kowalko, J.E. and Pipan, T. | Journal Article | 2023 | Natural selection versus neutral mutation in the evolution of subterranean life: A false dichotomy? |
| Kozol, R.A., Yuiska, A., Han, J.H., Tolentino, B., Lopatto, A., Lewis, P., Paz, A., Keene, A.C., Kowalko, J.E. and Duboue, E.R. | Journal Article | 2023 | Novel husbandry practices result in rapid rates of growth and sexual maturation without impacting adult behavior in the blind Mexican cavefish |
| Batista da Silva, I., Barbosa, D.A., Kavalco, K.F., Nunes, L.R., Pasa, R. and Menegidio, F.B. | Journal Article | 2023 | Discovery of putative long non‑coding RNAs expressed in the eyes of Astyanax mexicanus (Actinopterygii: Characidae) |
| Jiménez, A.G., Nash-Braun, E. and Meyers, J.R. | Journal Article | 2023 | Chronic thermal acclimation effects on critical thermal maxima (CTmax) and oxidative stress differences in white epaxial muscle between surface and cave morphotypes of the Mexican cavefish (Astyanax mexicanus) |
| Gil Galvez, A. | Thesis | 2023 | The role of Cis-Regulatory elements during evolution at different evolutionary time points |
| Baumann, D.P. and Ingalls, A. | Book Section | 2023 | Cryopreservation of the Mexican Tetra Astyanax mexicanus |
| Yang, X., Song, Y., Zhang, R., Yu, M., Guo, X., Guo, H., Du, X., Sun, S., Li, C., Mao, X., Fan, G. and Liu, X. | Journal Article | 2023 | Unraveling the genomic features, phylogeny and genetic basis of tooth ontogenesis in Characiformes through analysis of four genomes |
| Lakhiani, R., Shanavas, S. and Melnattur, K. | Journal Article | 2023 | Comparative biology of sleep in diverse animals |
| Berning, D. and Gross, J.B. | Journal Article | 2023 | The constructive evolution of taste in Astyanax cavefish: A review. |
| Olsen, L., Krishnan, J., Banks, C., Hassan, H. and Rohner, N. | Journal Article | 2023 | Circadian rhythm disruption is associated with skeletal muscle dysfunction within the blind Mexican cavefish |
| Blin, M., Rétaux, S. and Torres-Paz, J. | Book Section | 2023 | Whole-mount multicolor fluorescent labeling by In situ hybridization in Astyanax mexicanus embryos and larvae |
| Legendre, L., Rode, J., Germon, I., Pavie, M., Quiviger, C., Policarpo, M., Leclercq, J., Père, S., Fumey, J. Hyacinthe, C. et al. | Journal Article | 2023 | Genetic identification and reiterated captures suggest that the Astyanax mexicanus El Pachón cavefish population is closed and declining |
| Castillo-Casas, J.M., Caño-Carrillo, S., Sánchez-Fernández, C., Franco, D. and Lozano-Velasco, E. | Journal Article | 2023 | Comparative analysis of heart regeneration: Searching for the key to heal the heart—Part I: Experimental injury models to study cardiac regeneration |
| Gross, J.B. and Powers, A.K. | Journal Article | 2023 | Reinterpreting the work of Charles Breder: Sensory neuromasts and orbital skeleton variation in eyeless Astyanax cavefish |
| Kuball, K., Fernandes, V.F.L, Takagi, D. and Yoshizawa, M. | Journal Article | 2023 | Blind cavefish evolved food-searching behavior without changing sensory modality compared with sighted conspecies in the dark |
| Cardozo, M.J., Sánchez-Bustamante, E. and Bovolenta, P. | Journal Article | 2023 | Optic cup morphogenesis across species and related inborn human eye defects |
| Sifuentes-Romero, I., Ferrufino, E. and Kowalko, J.E. | Book Section | 2023 | Application of CRISPR-Cas9 for functional analysis in A. mexicanus |
| Blin, M., Valay, L., Kuratko, M., Pavie, M. and Rétaux, S. | Journal Article | 2023 | Evolution of olfactory sensitivity, preferences and behavioral responses in Mexican cavefish: fish personality matters |
| Hamm, A.R. | Thesis | 2023 | Understanding the integration between the sensory and skeletal systems: The lateral line and facial skeleton of the Mexican tetra, Astyanax mexicanus |
| Hyacinthe, C., Attia, J., Schutz, E., Lego, L., Casane, D. and Retaux, S. | Journal Article | 2023 | Acoustic signatures in Mexican cavefish populations inhabiting different caves |
| Arcila, D., Rincon-Sandoval, M., Hanson, W., Hart, P.B., González, V.L., Betancur-R, R. and Bichuette, M.E. | Journal Article | 2023 | Transcriptomic analysis of the Brazilian blind characid, Stygichthys typhlops, reveals convergent selection with Astyanax mexicanus and other cavefishes |
| Olsen, L. plus 17 others | Journal Article | 2023 | Metabolic reprogramming underlies cavefish muscular endurance despite loss of muscle mass and contractility |
| Lloyd, E., Kozol, R.A., Duboue, E.R. and Keene, A.C. | Book Section | 2023 | Immunohistochemistry and in vivo neural imaging in A. mexicanus |
| Iwashita, M., Tran, A., Garcia, M., Cashon, J., Burbano, D., Salgado, V., Hasegawa, M., Balmilero‑Unciano, R., Politan, K., Wong, M., Lee, R.W.Y. and Yoshizawa, M. | Journal Article | 2023 | Metabolic shift toward ketosis in asocial cavefish increases social‑like affinity |
| Weinberger, M. and Riley, P.R. | Journal Article | 2023 | Animal models to study cardiac regeneration |
| Moran, R.L., Richards, E.J., Ornelas-García, C.P., Gross, J.B., Donny, A., Wiese, J., Keene, A.C., Kowalko, J.E., Rohner, N. and McGaugh, S.E. | Journal Article | 2023 | Selection-driven trait loss in independently evolved cavefish populations |
| Biswas, T., Rajendran, N., Hassan, H., Zhao, C. and Rohner, N. | Journal Article | 2023 | 3D spheroid culturing of Astyanax mexicanus liver-derived cell lines recapitulates distinct transcriptomic and metabolic states of in vivo tissue environment |
| Gross, J.B., Berning, D., Phelps, A. and Luc, H. | Journal Article | 2023 | An analysis of lateralized neural crest marker expression across development in the Mexican tetra, Astyanax mexicanus |
| Hutton, P., Lloyd, E., Dotson, M. and Keene, A.C. | Book Section | 2023 | Sleep behavior analysis in Astyanax mexicanus |
| Alunni, A., Pierre, C., Torres-Paz, J., Clairet, N., Langlumé, A., Pavie, M., Escoffier-Pirouelle, T., Leblanc, M., Blin, M. and Rétaux, S. | Journal Article | 2023 | An Astyanax mexicanus mao knockout line uncovers the developmental roles of monoamine homeostasis in fish brain |
| Wilkens, H. | Journal Article | 2023 | The general significance of variability in cave regressive traits for evolution |
| Staeger, S. | Thesis | 2023 | Investigating neuroanatomical evolution through proliferative cells in the Astyanax mexicanus |
| Espinasa, L., Pavie, M. and Rétaux, S. | Journal Article | 2023 | Protocol for lens removal in embryonic fish and its application on the developmental effects of eye regression |
| Atukorallaya, D., Bhatia, V. and Gonzales, J. | Journal Article | 2023 | Divergent tooth development mechanisms of Mexican tetra fish (Astyanax mexicanus) of Pachon cave origin |
| Iwashita, M. and Yoshizawa, M. | Book Section | 2023 | A new method to analyze nonvisual-based social-like interactions in asocial cave fish |
| Boyle, M.J., Arledge, S., Thomas, B., Tomkins, J.P. and Guliuzza, R.J. | Conference Paper | 2023 | Testing the cavefish model: An organism-focused theory of biological design |
| Strand, S.H. | Web Page | 2023 | The impact of temperature on developmental morphology and LR Asymmetry in Astyanax mexicanus |
| Legendre, L., Espinasa, L., Lacaille-Múzquiz, J.-L., Alaniz-Garfia, G., Ornelas-García, P. and Rétaux, S. | Journal Article | 2023 | First record of a freshwater cave sponge (Porifera, unknown gen. and sp.) in a cave inhabited by Astyanax cavefish in the Sierra de El Abra, San Luis Potosí, Mexico |
| Olsen, L., Krishnan, J., Banks, C., Hassan, H. and Rohner, N. | Journal Article | 2023 | Circadian rhythm disruption linked to skeletal muscle dysfunction in the Mexican Cavefish |
| Olsen, L., Alexander, R., Krishnan, J. and Rohner, N. | Book Section | 2023 | A generalist’s guide to quantifying swimming behavior within traditional and nontraditional teleost models |
| Powers, A.K., Hyacinthe, C., Riddle, M.R., Kim, Y.K., Amaismeier, A., Thiel, K., Martineau, B., Ferrante, E., Moran, R.L., McGaugh, S.E., Boggs, T.E., Gross, J.B. and Tabin, C.J. | Journal Article | 2023 | Genetic mapping of craniofacial traits in the Mexican tetra reveals loci associated with bite differences between cave and surface fish |
| Yang, Y., Xu, T., Conant, G., Kishino, H., Thorne, J.L and Ji, X. | Journal Article | 2023 | Interlocus gene conversion, Natural Selection, and Paralog Homogenization |
| Sifuentes-Romero, I., Aviles, A.M., Carter, J.L., Chan-Pong, A., Clarke, A., Crotty, P., Engstrom, D., Meka, P., Perez, A., Perez, R., Phelan, C., Sharrard, T., Smirnova, M.I.,Wade, A.J. and Kowalko, J.E. | Journal Article | 2023 | Trait loss in evolution: What cavefish have taught us about mechanisms underlying eye regression |
| Frøland Steindal, A., Yamamoto, Y. and Whitmore, D. | Journal Article | 2023 | Blind fish have cells that see light |
| Gross, J.B., Boggs, T.E., Rétaux, S. and Torres-Paz, J. | Book Section | 2023 | Chapter 15. Developmental and genetic basis of troglomorphic traits in the teleost fish Astyanax mexicanus |
| Hyacinthe, C. | Book Section | 2023 | Recording acoustic behavior in Astyanax mexicanus fish: Acquisition, decryption, and interpretation |
| Riddle, M., Nguyen, N., Nave, M., Peuß, R., Maldonado, E., Rohner, N. and Tabin, C. | Journal Article | 2023 | Genetic adaptation shapes gut microbiome composition in Astyanax mexicanus |
| Rétaux, S. and Jeffery, W.R. | Book Section | 2023 | Chapter 12. Voices from the underground: animal models for the study of trait evolution during groundwater colonization and adaptation |
| Espinasa, L., Diamant, R., Vinepinsky, E. and Espinasa, M. | Journal Article | 2023 | Evolutionary modifications of Astyanax larval prey capture (LPC) in a dark environment |
| Enriquez ,M.S., Swanson, N., Putland, R.L., Tait, T., Gluesenkamp, A.G., McGaugh, S.E. and Mensinger, A.F. | Journal Article | 2023 | Evidence for rapid divergence of sensory systems between Texas populations of the Mexican tetra (Astyanax mexicanus) |
| Miranda-Gamboa, R., Espinasa, L., Verde-Ramírez, M.A., Hernández-Lozano, J., Lacaille, J.L., Espinasa, M., Ornelas-García, C.P. | Journal Article | 2023 | A new cave population of Astyanax mexicanus from Northern Sierra de El Abra, Tamaulipas, Mexico |
| Espinasa, L. and Lewis, K.A. | Journal Article | 2023 | Eye convergence is evoked during larval prey capture (LPC) without visual stimulus and in blind cavefish |
| Ma, L., Yang, J.X., Lei, F.K., Xu, M.Z., Zhao, Y.H. and Jeffery, W.R. | Journal Article | 2023 | Protection and exploration of the scientific potential of Chinese cavefish |
| Perera, P.P., Guerra, D.P. and Riddle, M.R. | Journal Article | 2023 | The Mexican Tetra, Astyanax mexicanus, as a model system in cell and developmental biology |
| Jeffery, W.R., Ma. L. and Zhao, Y.H. | Journal Article | 2023 | Cavefish as biological models in the laboratory and in the wild |
| Olsen, L., Hassan, H., Keaton, S. and Rohner, N. | Journal Article | 2023 | The Mexican cavefish mount a rapid and sustained regenerative response following skeletal muscle injury |
| Rajendran, N., Deng, F., Wang, Y., Kenzior, O., Krishnan, J., Biswas, T., Parmely, T., Rohner, N., Zhao, C. | Journal Article | 2023 | Establishment, long-term maintenance, and characterization of primary liver cells from Astyanax mexicanus |
| Stowers Institute | Web Page | 2023 | Stowers scientists use cavefish to learn about metabolism and the evolutionary basis of being a couch potato |
| Hildebrandt, M., Kotewitsch, M., Kaupp, S., Salomon, S., Schuster, S. and Machnik, P. | Journal Article | 2024 | Stabilizing selection in an identified multisensory neuron in blind cavefish |
| Boggs, T. | Thesis | 2024 | Adaptation to hypoxia in the blind Mexican cavefish, Astyanax mexicanus |
| Ullah, K., Rani, R., Shaheen, F. and Tasleem, F. | Journal Article | 2024 | Teleost caudal fin development and regeneration: Mechanisms, models, and therapeutic potential |
| Di Lorenzo, T., Mori, M. and Simčič, T. | Journal Article | 2024 | Metabolic rates of groundwater species as a function of body mass and temperature |
| Boggs, T.E. and Gross, J.B. | Journal Article | 2024 | Gill morphology adapted to oxygen‐limited caves in Astyanax mexicanus |
| Biswas, T., Hassan, H. and Rohner, N. | Journal Article | 2024 | Potential role of microRNAs in regulating transcriptional profile, and sculpting development and metabolism in cavefish |
| Biswas T., Rajendran N., Hassan H., Li H., Zhao C. and Rohner N. | Journal Article | 2024 | 3D spheroid culturing of Astyanax mexicanus liver-derived cell lines recapitulates distinct transcriptomic and metabolic states of in vivo tissue environment. |
| Silva, M., Mazzoni Zerbinato Andrade Silva, D., Castro, J.P., Makunin, A.I., Barby, F.F., de Oliveira, E.H.C. | Journal Article | 2024 | Investigation of Astyanax mexicanus (Characiformes, Characidae) chromosome 1 structure reveals unmapped sequences and suggests conserved evolution |
| Di Lorenzo, T., Mori, N. and Simčič, T. | Web Page | 2024 | Dataset for “Metabolic rates of groundwater species as a function of body mass and temperature” |
| Alkafaween, E., Hassanat, A., Essa, E. and Elmougy, S. | Journal Article | 2024 | CSGA: A dual population Genetic Algorithm based on Mexican Cavefish genetic diversity |
| Swaminathan, A., Xia, F. and Rohner, N. | Journal Article | 2024 | From darkness to discovery: evolutionary, adaptive, and translational genetic insights from cavefish |
| Toledo Piza and 48 other authors | Journal Article | 2024 | Checklist of the species of the Order Characiformes (Teleostei: Ostariophysi) |
| Hernandez Lozano, J., Garita Alvarado, C.A., Munguia Stayer, R., Garduno Sanchez, M.A. and Ornelas Garcia, C.P. | Journal Article | 2024 | Parallel phenotypic evolution of two independent cavefish lineages of Astyanax mexicanus (De Filippi, 1854) (Characiformes: Characidae) |
| Manning, A.E., Grunwald, H., Moran, R., Rodriguez-Morales, R., Powers, A.K., McGaugh, S. and Kowalko, J.E. | Journal Article | 2024 | Defining the unseen: Population-specific markers for Astyanax mexicanus blind cavefish |
| Accorsi, A., Guo, L., Marshall, W.F., Mommersteeg, M.T.M. and Nakajima, Y.-I. | Journal Article | 2024 | Extraordinary model systems for regeneration |
| Espinasa, L., Tatarsky, R.L., Girard, M.K., Sandone, M., Rétaux, S. and Espinasa, J. | Journal Article | 2024 | Population size and spatial distribution of the Mexican blind cavefish (Astyanax) within the caves |
| Pezer, Z., Pokrovac, I. and Rohner, N. | Journal Article | 2024 | Increase in copy number at multiallelic copy number variants facilitates rapid adaptation to subterranean habitats in Mexican Astyanax fishes |
| Lunghi E. and Bilandžija H. | Journal Article | 2024 | Telomere length and dynamics in Astyanax mexicanus cave and surface morphs |
| Blin, M., Valay, L., Kuratko, M., Pavie, M. and Rétaux, S. | Journal Article | 2024 | The evolution of olfactory sensitivity, preferences, and behavioral responses in Mexican cavefish is influenced by fish personality. |
| Turner, G.F. | Journal Article | 2024 | Cladistic species definitions can lead to under-representation of biodiversity from adaptive radiations |
| Keene, A.C., Duboue, E.R., Foulkes, N.S. and Bertolucci, C. | Book Section | 2024 | Evolved loss of sleep and circadian rhythms in cavefish |
| Juan, T. and Ebnicher, G. | Journal Article | 2024 | In preprints: Shh signaling activity predicts cardiac laterality in Astyanax mexicanus populations |
| Rodríguez-Morales, R. | Journal Article | 2024 | Sensing in the dark: Constructive evolution of the lateral line system in blind populations of Astyanax mexicanus |
| Kuball, K., Fernandes, V.F.L., Takagi, D. and Yoshizawa, M. | Journal Article | 2024 | Blind cavefish evolved higher foraging responses to chemo- and mechanostimuli |
| Rétaux, S. and Yamamoto, K. | Journal Article | 2024 | Evolution of fish brains and behaviors: how many ways to generate the same outcomes? |
| Espinasa, J. and Espinasa, L. | Journal Article | 2024 | Cavefish dorsoventral axis angle during wall swimming: laterality asymmetry |
| Brown, E.A. | Web Page | 2024 | Deze blinde vis heeft smaakpapillen op zijn kop en kin |
| Fang, Z. | Thesis | 2024 | Investigating dentition development and replacement in Mexican tetra two subforms |
| Choy, S., Thakur, S., Polyakov, E., Abdelaziz, J., Lloyd, E., Enriquez, M., Jayan, N., Fily, Y., McGaugh, S., Keene, A.C., Kowalko, J.E. | Journal Article | 2024 | Mutations in the albinism gene oca2 alter vision-dependent prey capture behavior in the Mexican tetra |
| Pozo-Morales, M., Cobham, A.E., Centola, C., McKinney, M.C., Liu, P., Perazzolo, C., Lefort, A., Libert, F., Bai, H. Rohner, N. and Singh, S.P. | Journal Article | 2024 | Starvation resistant cavefish reveal conserved mechanisms of starvation induced hepatic lipotoxicity |
| Wiese, J., Richards, E., Kowalko, J.E. and McGaugh, S.E. | Journal Article | 2024 | Quantitative trait loci concentrate in specific regions of the Mexican cavefish genome and reveal key candidate genes for cave-associated evolution |
| Ng, M., Ma, L., Shi, J. and Jeffery, W.R. | Journal Article | 2024 | Natural reversal of cavefish heart asymmetry is controlled by Sonic Hedgehog effects on the left-right organizer |
| Cobham, A.E. and Rohner, N. | Journal Article | 2024 | Unraveling stress resilience: Insights from adaptations to extreme environments by Astyanax mexicanus cavefish |
| Melo, B.F., Ota, R.P., Benine, R.C., Carvalho, F.R., Lima, F.C.T., Mattox, G.M.T., Souza, C.S., Faria, T.C., Reia, L., Roxo, F.F., Valdez-Moreno, M., Near, T.J., et al. | Journal Article | 2024 | Phylogenomics of Characidae, a hyper-diverse Neotropical freshwater fish lineage, with a phylogenetic classification including four families (Teleostei: Characiformes) |
| Tidswell, B.K. | Thesis | 2024 | Vision and the lateral line: Comparative behavioral studies of fish schooling |
| Berning, D., Heerema, H. and Gross, J.B. | Journal Article | 2024 | The spatiotemporal and genetic architecture of extraoral taste buds in Astyanax cavefish |
| Gallman, K., Rastogi, A., North, O., O'Gorman, M., Hutton, P., Lloyd, E., Warren, W.C., Kowalko, J.E., Duboue, E.R., Rohner, N. and Keene, A.C. | Journal Article | 2024 | Postprandial sleep in short‐sleeping Mexican cavefish |
| Folgueira, M. and Clarke, J.D.W. | Journal Article | 2024 | Telencephalic eversion in embryos and early larvae of four teleost species |
| Cardoso, A.L., Oliveira, J.I.N., Climaco, J.P.S., Venturelli, N.B., Moreira, D.D.N. and Martins, C. | Journal Article | 2024 | Conditions for establishing fin primary cell cultures in a wide range of ray‑finned fishes |
| Pozo‐Morales, M., A. E. Cobham, C. Centola, et al. | Journal Article | 2024 | Starvation‐resistant cavefish reveal conserved mechanisms of starvation‐induced hepatic lipotoxicity |
| Di Rosa, V., Frigato, E., Negrini, P., Cristiano, W., López-Olmeda, J.F., Rétaux, S., Sánchez-Vázquez, F.J., Foulkes, N.S., Bertolucci, C. | Journal Article | 2024 | Sporadic feeding regulates robust food entrainable circadian clocks in blind cavefish |
| Policarpo, M., Legendre, L., Germon, I., Espinasa, L., Rétaux, S., Casane, D. and Lafargeas, P. | Journal Article | 2024 | The nature and distribution of non-functional alleles suggest only two independent events at the origins of Astyanax mexicanus cavefish populations |
| Marketaki, S.Z., Berio, F. and Di Santo, V. | Journal Article | 2025 | Compensatory sensory mechanisms in naïve blind cavefish navigating novel environments after lateral line ablation |
| Xia, F., Santacruz, A., Wu, D., Bertho, S., Fritz, E., Morales-Sosa, P., McKinney, S., Nowotarski, S.H. and Rohner, N. | Journal Article | 2025 | Reproductive adaptation of Astyanax mexicanus under nutrient limitation |
| Knight, K. | Journal Article | 2025 | Ghostly gene helps blind cave fish hunt better in the dark (Commentary article refering to Choy et al. 2025) |
| Choy, S., Thakur, S., Polyakov, E., Abdelaziz, J., Lloyd, E., Enriquez, M., Jayan, N., Fily, Y., McGaugh, S., Keene, A.C., Kowalko, J.E. | Journal Article | 2025 | Mutations in the albinism gene oca2 alter vision-dependent prey capture behavior in the Mexican tetra |
| Powers, A.K., Amaismeier, A., Thiel, K., Anyonge, W., McGaugh, S.E., Boggs, T.E., Tabin, C.J. and Gross, J.B. | Journal Article | 2025 | Genetic mapping of orofacial traits reveals a single genomic region associated with differences in multiple parameters of jaw size between Astyanax mexicanus surface and cavefish |
| Legendre, L., Père, S., Rebaudo, F., Espinasa, L. and Rétaux, S. | Journal Article | 2025 | Water parameters and hydrodynamics in rivers and caves hosting Astyanax mexicanus populations reveal macro-, meso- and micro-habitat characteristics |
| Biswas, T., Li, H. and Rohner, N. | Journal Article | 2025 | Divergent 3D genome organization links to metabolic adaptation in cavefish |
| Brachmann, M.K., Costa, A.P.B., Robertson, S., Donoghue, K., Pilakouta, N., Whitehead, M., Liu, X., Kristjansson, B., Skulason, S., Selman, C. and Parsons, K. | Journal Article | 2025 | Parallel adaptation to geothermally-warmed habitats due to common structural variation and functional developmental pathways |
| Fang, Z. and Atukorallaya, D. | Journal Article | 2025 | Exploration of dentition development and replacement in two forms of Mexican Tetra (Astyanax mexicanus) |
| Huang, S., Hui, W., Kan, T., Yu, H., Liang, X., Liang, Y., Zhou, Z. and and Feng, P. | Journal Article | 2025 | Complex association between diet and bitter taste receptor gene numbers in vertebrate from an enlarged sampling |
| Perera, P.P., Webster, K. and Riddle, M.R. | Journal Article | 2025 | Enteric neural crest development in Astyanax mexicanus surface fish and cavefish |
| Hamm, A.R. and Gross, J.B | Journal Article | 2025 | Integration of the sensory and skeletal systems: A classical perspective on neuromast-bone interactions |
| Cobham, A., Li, H., Acheampong, D., McKinney, C. and Rohner, N. | Web Page | 2025 | Understanding the mechanisms of adaptation to altered glucose homeostasis in the blind cavefish (Astyanax mexicanus ) |
| Hernández-Lozano, J., Córdova-Tapia, F., Miranda-Gamboa, R., Vázquez-Cruz, MdeL., Garita-Alvarado, C., Mercado-Silva, N., Ornelas-García, C.P. | Journal Article | 2025 | Isotopic variability of δ13C and δ15N signals in cave systems: insights from the blind tetra Astyanax mexicanus. |
| Biswas, T., Hassan, H. and Rohner, N. | Journal Article | 2025 | Differentially expressed miRNAs offer new perspective into cave adaptation of Astyanax mexicanus |
| Bauhus, M.B., Wander, L., Boy, D., Santacruz, A., Maldonado, E., Kurtz, J., Kaiser, S. and Peuß, R. | Journal Article | 2025 | Environmental dynamics influence scale cortisol levels in Mexican cavefish |
| Boggs, T.E. and Gross, J.B. | Journal Article | 2025 | Elevated blood hemoglobin in different cavefish populations evolves through diverse hemoglobin gene expression patterns |
| Sekulovski, B. and Miller, N. | Journal Article | 2025 | Mechanisms of social behaviour in the anti-social blind cavefish (Astyanax mexicanus) |
| Conti, F., Verges-Castillo, A., J. Sanchez-Vazquez, F.J., Lopez-Olmeda, J.F., Bertolucci, C., Munoz-Cueto, J.A. | Journal Article | 2025 | Daily rhythms of locomotor activity and transcript levels of non-visual opsins in the brain of the blind Mexican cavefish (Astyanax mexicanus) |
| Roback. E.Y., Ferrufino, E., Moran, R.L., Shennard, D., Mulliniks, C., Gallop, J., Weagley, J., Miller, J., Fily, Y., Ornelas-García, C.P., Rohner, N., Kowalko, J.E. and McGaugh, S.E. | Journal Article | 2025 | Population genomics of premature termination codons in cavefish with substantial trait loss |
| Padmanaban, N., Ambosie. R., Choy, S., Marcus, S., Nilsson, S.R.O., Keene, A.C., Kowalko, J.E. and Duboué, E.R. | Journal Article | 2025 | Automated behavioral profiling using neural networks reveals differences in stress-like behavior between cave and surface-dwelling Astyanax mexicanus |